There continues to be renewed focus on waterborne alkyd resins due to advancements in their chemistry, as well as increased interest in green chemistry. This has led to new waterborne alkyd resins and coatings that offer many of the attractive performance features of older alkyd resins while also providing some additional benefits. Of course these newer resins pose different formulation, application and performance challenges, some of which are due to different surface chemistry properties. This can lead to different surface wetting, flow and leveling characteristics, as well as a tendency to foam. These traits, if not addressed adequately, lead to coating application and performance deficiencies. This article describes the selection of optimal defoamer and wetting agent additives for use with newer waterborne alkyd resin technology and related coating formulations. Recommendations for specific additives are provided, along with associated performance benefits.
Introduction
Alkyd resins are prepared from the polycondensation reaction of a polyhydric alcohol with a polybasic acid, and modified with an oil or fatty acid (i.e., natural oils). They can be further modified with a variety of other polymer chemistries such as polyurethane, acrylic, silicone and epoxy esters to tailor and obtain specialized properties. Recently, water-based alkyd resin technology has expanded with a variety of different chemistries and morphologies.(1-5) One example is the development of waterborne resins containing particles with an alkyd core surrounded by an acrylic shell to provide attractive features of both polymers, such as the long shelf life, quick drying, good weathering of acrylics, and the crosslinking and high gloss of alkyds.
Oxidizing alkyd resins cure by the oxidation of the unsaturated fatty acid ester chains in the alkyd. These reactions are accelerated with metal-based catalysts, also known as driers. This drying reaction occurs after application and yields a crosslinked applied coating. The wide variety of available chemistries to produce and modify alkyds, along with their oxidative crosslinking cure, results in a broad range of attractive properties, from soft and flexible to hard and brittle. They also are characterized by good adhesion, high gloss and chemical resistance. References 6 and 7 provide further description of alkyd resins and their use in coatings.
Alkyd resins can be designed for use in solvent-based or waterborne coatings. Waterborne versions can be water reducible or dispersions. Water-reducible alkyds are solubilized in water and cosolvent by neutralizing the acid groups on the alkyd with amine(s). They are typically formulated at 25-35% solids with VOCs of > 200 g/L. Waterborne alkyd dispersions are newer technology, where alkyd resins are dispersed as particles in water. They can be formulated at 40-60% solids, with VOCs of < 120 g/L, with some formulations well under 50 g/L. This offering of extremely low VOCs, along with the use of “green” raw materials (i.e., natural oils), also makes waterborne alkyds attractive from an environmental, health and safety standpoint.
Alkyd resin properties make them suitable for interior and exterior architectural coatings, especially wood paints and stains, as well as industrial metal primers and topcoats. Waterborne alkyd coating formulations have ingredients similar to most other waterborne coatings. The alkyd resin serves as film former and binder. Specific alkyd chemistry and type are selected based on the end-use application and desired properties. Other typical formulation ingredients include metal driers (noted above), pigments and fillers (for color coats), co-solvent, wetting agents, defoamers, rheology modifiers and other additives.
Although ingredient types in waterborne alkyd coatings generally are similar to those with other waterborne resins (e.g., acrylic latex, polyurethane dispersions, waterborne epoxy, etc.), the specific types and combinations of ingredients, mixing styles and procedures, applications and cures are somewhat different. This poses unique application and film characteristics, especially those related to surface chemistry, such as flow, leveling and foam. For example, waterborne alkyd resins and coatings can generate substantial foam when agitated during manufacturing, mixing and application processes. This foam can lead to a variety of difficulties that can be avoided with the proper selection and incorporation of suitable anti-foam and defoamer additives. The work described below was undertaken to study the flow, leveling and foam characteristics of waterborne alkyd resins and coatings, and to identify optimal defoamers and wetting agents for waterborne alkyd formulations.
Basics of Wetting and Defoaming
Due to the high surface tension of water, waterborne coatings typically require surfactants to reduce surface tension and improve wetting and leveling of the applied coating, especially on low-energy surfaces such as wood, plastic, contaminated metal and previously coated surfaces. Surfactants reduce surface tension by diffusing to, concentrating, and aligning at an interface such as the coating/substrate and coating/air interfaces. References 8-10 discuss surface tension, substrate wetting and the use of surfactants in greater detail. An additional consideration is that many coating-related processes, such as paint manufacture (e.g., pigment dispersion), mixing and application, are high-speed agitation processes.
These processes disrupt the surfactant concentration and alignment at the surface, thereby causing higher surface tension during and after agitation, which is also known as dynamic surface tension (DST). Surfactants that quickly diffuse back to the interface, align and lower surface tension have low DST, i.e., they provide low surface tension during and shortly after these dynamic agitating processes. They are effective and efficient at providing good wetting and leveling, and avoiding problems such as craters, fisheyes and other defects. Gemini (twin) surfactants have proven their efficiency and effectiveness at reducing surface tension in waterborne coating formulations and applications to obtain superior film formation and performance. They obtain this low DST due to their unique chemical structure, which contains two hydrophobic and hydrophilic groups in a single molecule, thus being more efficient than typical surfactants that contain a single hydrophobe/hydrophile structure.11,12
Waterborne coatings also have a tendency to foam when agitated, which occurs during every manufacturing, mixing and application process. Foam can be very tenacious and last for hours or even days, and it can cause numerous problems in coating production, application, film formation, and throughout the coating’s service life. Foam can reduce pigment grinding efficiency and increase production time and energy demand; it can also lower product density leading to incomplete filling of production vessels and final product packaging. Foam also can create problems during application by reducing the efficiency of transferring the product to the substrate and by creating defects in the dry film that reduce the protective and aesthetic qualities of the finished product.
To avoid these problems, defoamers are commonly used in waterborne coatings; they can be very effective but they need to be carefully selected. In general, defoamers work by disrupting the surfactant “bi-layer” around the air bubble, destabilizing the bubble wall so that the bubble can break and release the trapped air. Conventional defoamers such as silicones and mineral and other organic oil-based defoamers are insoluble, low-surface-tension materials that are capable of entering and spreading across bubble surfaces. They displace the stabilizing surfactants and form an unstable film at the bubble surface that easily ruptures, breaking the bubble and collapsing the foam.
Molecular defoamers have a different defoaming mechanism than conventional defoamers. They work by replacing foam-stabilizing surfactants at the bubble surface on a molecular level.13 Molecular defoamers are particularly effective at controlling microfoam (small bubbles <70µm, trapped within the liquid) because they are partially soluble in water and are available to move to bubbles below the liquid surface to help de-aeration. They can also provide additional dynamic wetting properties to the formulation.11, 14
Selection and performance of wetting agents and defoamers are complicated further by their possible competition within a formula. As mentioned above, conventional defoamers work by an incompatibility mechanism. Therefore, conventional silicone and mineral oil defoamers, if not selected and incorporated properly, can cause surface defects such as craters by forming localized areas of very low surface tension, which do not wet, thus forming craters. Formulators who experience this often utilize higher concentrations of wetting agent to induce better, more complete surface wetting. But many surfactants generate and stabilize foam, which then requires more defoamer. This potential competition between defoamer and wetting agent can cause this difficult cycle.
There are a large variety of wetting agents and defoamers that can be used in coating formulations. The combination of these two additive types leads to an extreme number of formulation possibilities. The performance of these additives is highly influenced by the specific coating chemistry and how the coating is prepared and applied. Therefore, selection of proper defoamers and wetting agents for optimal coating performance is critical yet complex, so selection can be difficult.15 This applies to alkyd resins and coatings as well. The following studies were performed to assess and select defoamers and wetting agents that will provide optimal performance in waterborne alkyd resin systems and coatings.
Experimental
Resins, Formulations and Wet Coating Properties
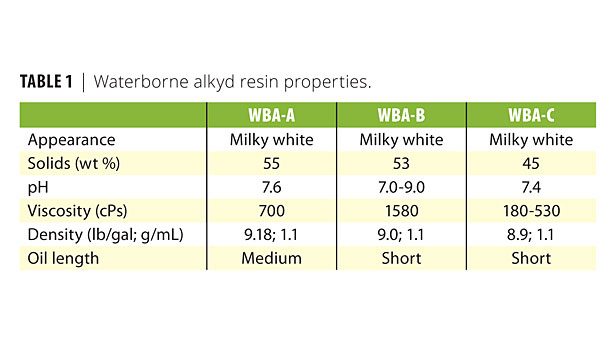
To assess a variety of waterborne alkyd resin types and chemistries, seven commercial samples were obtained from four separate manufacturers. Suggested starting coating formulations for these resins also were obtained from their suppliers. Although all the resins were evaluated with defoamer and wetting agent additives, the work described here focuses on three resins and associated formulations from three separate suppliers. While these resins are used in somewhat different applications, their results are representative of what was observed with the entire set of seven resins studied. The three resins discussed here are designated WBA-A, WBA-B and WBA-C, respectively.
Table 1 lists their typical properties. WBA-A and WBA-B initially were evaluated as received (i.e., adding only defoamer and/or wetting agent). For these resin/additive mixtures, the additives were incorporated slowly into the resin while being gently mixed for 10 min with a propeller blade, creating a small vortex. Additive concentration in this phase was 53.3 g of resin to 0.11 g of additive (0.2%). These samples were equilibrated overnight and then density (foam assessment) was measured after vigorously shaking the resin/defoamer mixtures by hand for 1 min.
Following the above resin screening phase, all three resins were evaluated in the control formulations provided by their respective suppliers as listed in Tables 2-4. The formulations were prepared per the ingredients and procedures provided in the resin manufacturers’ starting formulations, including the listed defoamers and wetting agents (these formulations are designated as “control”). A series of additional formulations were prepared similarly by substituting other silicone, mineral oil and molecular defoamers, as well as candidate wetting agents. Wet coating properties were characterized after the coatings were prepared and equilibrated.
They included pH, Stormer viscosity (Krebs Units, KU), viscosity after aging for 7 days at 50 oC (aging/storage stability), density and agitated (foam) density. As with the resin/additive mixtures, the agitated density for the WBA-A and WBA-B formulated coatings was obtained by measuring the paint density after vigorously shaking by hand for 1 min. The foam density for the WBA-C coating was measured using a Dispermat® foam test for an 80 g sample, stirred for 2 min at 4000 rpm. Density of the mixed sample was measured 30 sec after mixing (anti-foaming assessment) and 4 min after mixing (defoaming assessment).
Resin and Coating Application, Cure and Tests
Initially resins WBA-A and WBA-B were evaluated neat (i.e., not fully formulated), with only defoamer and wetting agent additives. These mixtures were applied to Leneta 5C charts with a 3 mil drawdown bar and with a 2-inch Purdy Sprig brush, respectively. The initial applications were performed after gentle stirring of the resin/additive mixtures to ensure homogeneity, and these are noted as “Stirred”. After these applications, the mixtures were shaken vigorously by hand for 1 min, then a second set of applications were made and these are noted as “Shaken”. After setting for several days, the applied coatings were evaluated for film quality and coating defects, i.e., flow, leveling, foam, craters, fisheyes, etc. A rating system of 0 (worst) – 10 (no defects) was used to characterize the coatings. In this manner, we assessed application and applied coating characteristics of un-agitated and agitated resin/additive mixtures.
In the next phase, formulated coatings with resins WBA-A, B and C were prepared, applied and evaluated. Coatings were prepared as described above and in Tables 2-4, and then applied to various substrates using drawdown bar and/or brush depending on the specific test to be performed. The WBA-A and WBA-B coatings were applied to Leneta 5C charts and yellow pine wood with drawdown bar and brush as described for the resin/additive mixtures above. After curing for 7 days at 70 oF, properties of the applied WBA-A and B coating were evaluated per the following procedures: gloss using a Hunter ProGloss glossmeter (ASTM D 523), contrast ratio using a Spectro-Glide 45/0 (ASTM D 2805), sag resistance (ASTM D 4400), scrub resistance using a Gardco D10 washability and wear tester (ASTM D 2186, method B), and cross cut tape adhesion to yellow pine (ASTM D 3359, method A).
The WBA-C coatings were applied to Leneta BK black charts and Q panel S36 I steel panels (SAE 1008/1010 ground; ASTM A1008, D 609 Type 2) using a 6 mil (150 micron) drawdown bar. The coatings on Leneta charts were evaluated for coating appearance and craters using a rating of 1 (worst) to 5 (best). After curing, the steel panels of the control coating and those with selected defoamers (those which were most promising after defoamer and appearance evaluations) were placed in an ASTM B117 5% salt spray and evaluated after 150 and 300 hours exposure durations.
Results and Discussion
Approximately 22 defoamer and wetting agent additives were evaluated in the waterborne alkyd resins and coatings throughout this study. The defoamers represented a variety of silicone, organic (mineral) oil and molecular defoamer types from several suppliers. The wetting agents evaluated generally were Gemini surfactants based on acetylenic chemistry. As the evaluations progressed, silicone defoamers, and particularly two silicone defoamers, Surfynol® DF-58 and Surfynol DF-66, clearly provided the best results related to foam density, wet and applied coating properties in all of the resins. Surfynol DF-75 defoamer, an organic oil-based defoamer, also performed well in the WBA-C primer formulation. In addition, two particular additives, Surfynol 420 and Dynol™ 607 surfactants provided benefits in some of the applications when used in combination with these defoamers. The results presented below focus on the performance provided by these additives, which are representative of the best results obtained and compared with the “control” formulations that contain the additives in the formulations provided by the resin suppliers.
Table 5 lists the results for the two best defoamers (DF-58 and DF-66), as well as the control defoamer and no defoamer in WBA-A. The first dramatic difference is the foam density of the agitated neat resin (0.63 g/mL) versus that for the resin/defoamer mixtures of ~1.00 g/mL. This clearly indicates the inherent tendency of this resin to foam. Furthermore, the foam for this neat resin was tenacious and lasted for hours. In fact, all of the waterborne alkyd resins evaluated had similar substantial foaming tendencies, which is common for waterborne alkyd resins. This tendency to foam is attributable to surfactants used in the resin synthesis, which stabilize foam as described previously. Most, but not all, of the defoamers evaluated improved foam density to ~1.0 g/mL (density of the neat unagitated resins was about 1.1 g/mL).
The two best performers from a foam density perspective are those listed in Table 5, DF-58 and DF-66, both of which are silicone-based. Table 5 also illustrates the application ratings for the drawdown and brush-applied coatings after gentle stirring and after vigorous shaking. These ratings illustrate tendencies of the applied coatings to display defects such as foam, craters, fisheyes, etc. Although the neat WBA-A resin looked nearly perfect with drawdown application after gentle stirring (rating = 10), the ratings for the brush-applied and shaken samples are much lower (i.e., 7 and 2 respectively) mainly due to foam trapped in the applied coating, which again illustrates the need for a defoamer for this resin system. The application ratings for WBA-A with the control defoamer also are poor, with ratings of < 5, and these low ratings are due to numerous large craters caused by the control defoamer. In this case, the control defoamer is extremely incompatible in the formulation and, when the coating is applied, creates numerous localized areas of very low surface tension, causing the coating to retract and form craters in these regions. The two best defoamers, and especially DF-58, have considerably higher ratings in all of the scenarios, including the most demanding of being applied immediately after vigorous shaking. Subsequent to these evaluations, the resin supplier evaluated this defoamer in a wood varnish clearcoat and confirmed excellent foam control, clarity, gloss and distinctness of image.
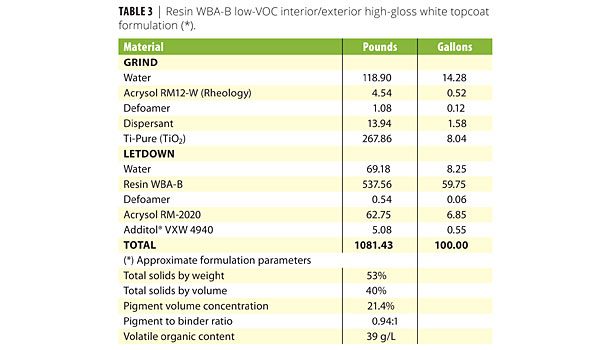
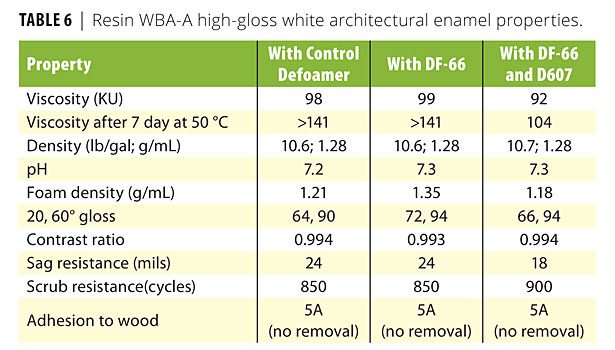
A variety of defoamers and wetting agents were then evaluated in formulated coatings containing WBA-A and WBA-B, as described in Tables 2 and 3. The WBA-A formulation is a high-gloss white interior wood trim coating. The WBA-B formulation is a high-gloss interior/exterior topcoat. Results for these coatings with the control defoamer and the best performing additives from those tested are listed in Tables 6 and 7, respectively. It should be noted that one of the major differentiating features of waterborne alkyd resins over other waterborne resins, including latex, is the ability to obtain high gloss with pigmented coatings, which strives to reach that of solventborne systems. Therefore, approaches to increase these gloss levels even further and improve wetting and appearance become an even greater benefit.
Tables 6 and 7 illustrate that the DF-66 in WBA-A and the DF-58 in WBA-B provide exceptional gloss, with substantial increases over the control formulations. For the WBA-A formulation, 20o and 60o gloss values increase from 64 and 90 in the control to 72 and 94 with DF-66. The DF-66 also improves foam density versus the control from 1.21 g/mL to 1.35 g/mL. The addition of D607, an alkoxylated acetylenic diol surfactant, along with DF-66 also increases gloss over the control. Although the gloss values do not reach the values obtained with just DF-66, appearance related to flow, leveling and distinctness of image were noticeably improved. D607 also improves heat age stability, which is reflective of aging and shelf life, by maintaining an applicable viscosity after 7 days at 50 oC. Other properties of the WBA-A coating, such as pH, contrast ratio, sag resistance, scrub resistance and adhesion, are comparable between the control defoamer and the DF-66 and DF-66 / D607 additives.
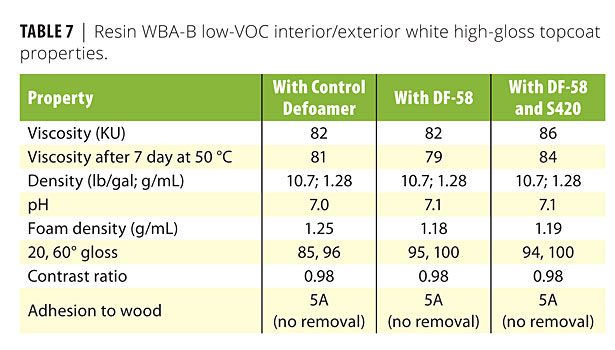
Table 7 provides results for the WBA-B interior/exterior high-gloss topcoat. In this formulation, use of DF-58 increased both 20o and 60o gloss values. The 20o gloss values increase from 85 to 95 with use of DF-58 defoamer. Considering that 20o gloss is a demanding value for high-gloss coatings, an increase of this magnitude and to this level (i.e., 95) is very significant. This is a notable differentiating feature for formulators trying to reach ultrahigh-gloss with low-VOC waterborne technology. It also should be noted that this formulation has a VOC of 39 g/L. As with the D607 wetting agent in the WBA-A coating, addition of S420, an ethoxylated acetylenic diol wetting agent, improves gloss, as well as visual appearance from flow, leveling and distinctness of image. Other coating properties are comparable with the control defoamer, DF-58 and DF-58 / S420.
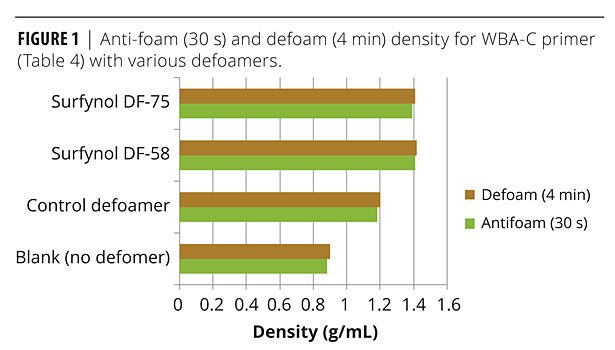
WBA-C is designed for metal applications, and most specifically corrosion preventive primers as illustrated by the control formulation listed in Table 4. A variety of alternate defoamers were evaluated in this formulation to assess defoaming, appearance (e.g., foam, crater formation), and corrosion protection. Foam density of the agitated samples with various defoamers and wetting agents ranged from 0.80 g/mL to 1.43 g/mL, with the un-agitated sample being approximately 1.49. Of these, the best additives were DF-58, DF-66 (silicone-based) and DF-75 (an organic oil). Figure 1 illustrates anti-foaming (30 s after mixing) and defoaming (4 min after mixing) density results with these additives, as well as the no “blank” defoamer formulation, and the control defoamer formulation. DF-58, DF-66 and DF-75 all provide both good anti-foaming and defoaming with densities close to or exceeding 1.4 g/mL, while the blank (no defoamer) is considerably lower (approximately 0.9 g/mL), and the control defoamer gives <1.2 g/mL.
The entire series of WBA-C coatings were applied to black Leneta charts to assess tendency to form craters. The blank, control, DF-58 and DF-75 all performed well by displaying continuous high- quality films with no crater indication. These coatings were then evaluated on steel panels in B117 5% salt spray. Figures 2 and 3 illustrate the 150 h and 300 h exposure results for these panels. At both exposure durations, the results clearly show considerable rust with the blank (no defoamer) and the control defoamer, compared to no rust with DF-58 and DF-75. The rust is caused by foam trapped within the applied coating, which creates pinholes and thin areas that are susceptible to corrosive contaminants attacking the steel substrate. In contrast, the DF-58 and DF-75 specimens show no signs of corrosion due to exceptional elimination of foam, thus preventing defects and resulting in a high-quality, continuous film from both an aesthetic and protective standpoint.
As mentioned above, seven waterborne alkyd resins were evaluated throughout this effort, with detailed results from only three discussed in detail in this article. Results with the other four resins were very similar in nature to those experienced with WBA-A, B and C resins and coatings formulations. Silicone defoamers DF-58 and/or DF-66 provided the best defoamer results in both the bulk wet coating and during application and cure of the applied coating. Additionally, wetting agents S420 and D607 also provided benefits, mainly better flow and leveling as expected. All of these coatings resulted in coatings with less defects, higher gloss and better appearance than their control counterparts (i.e., with the initially specified additives). These waterborne alkyd resins are recommended for a variety of architectural and industrial coating applications, including interior and exterior architectural primers and topcoat, wood interior and exterior (e.g., siding and decking) clearcoats and stains.
Summary
New waterborne alkyd resins offer a wide spectrum of compositions and properties, including very low VOCs (< 50 g/mL) and use of “green” raw materials, making them attractive from both a performance and environmental standpoint. These resins are suitable for a variety of applications, including interior and exterior architectural and industrial coatings. However, they also display somewhat different surface chemistry characteristics than other waterborne coatings. For example, many of these resins have a tendency to foam and, if unaddressed, this will lead to poor aesthetics (e.g., reduced gloss, haze, etc.) and protection (e.g., pinholes, corrosion protection). Therefore, defoamers are required for these resins and coatings to meet their optimum performance. However, not all defoamers provide the same level of defoaming and coating quality. Therefore, tangible benefits can be obtained by utilization of optimum defoamers. These coatings also can benefit from use of wetting agents to improve substrate wetting, flow and leveling to avoid surface defects and improve overall appearance, especially on difficult surfaces such as wood, poorly prepared metal and previously painted surfaces.
Seven waterborne alkyd resins with a variety of specific chemistries and properties were evaluated with 22 different defoamers and wetting agents in the neat resins, as well as in suggested starting formulations published by the resin manufacturers. Results of the various additives were compared for defoaming capability as well as applied coating appearance, optical and physical properties. Two particular silicone defoamers, Surfynol DF-58 and DF-66 surfactants, consistently provided significant improvements in the formulations. In particular, these defoamers increased gloss and appearance of a high-gloss architectural trim paint, as well as a high-gloss interior/exterior topcoat. Gloss values (20o) were increased 8 to 10 units, in one case up to 95, and 60o gloss up 4-6 units, up to 100. These gloss values rival those displayed by traditional high-gloss solventborne coatings. Gemini surfactant wetting agents Dynol 607 and Surfynol 420 surfactants also increased gloss and overall appearance by improving flow and leveling of the applied coating.
In a waterborne alkyd corrosion-preventive metal primer formulation, Surfynol DF-58 and DF-75 defoamers provided significantly better defoaming while leading to better coatings (i.e., no craters or other defects). They provided more dramatic improvements to corrosion protection as displayed in salt spray exposure. This was accomplished by eliminating or minimizing coating defects such as craters and foam, thereby improving coating quality and protecting the steel substrate from corrosive contaminants.
This work demonstrates the differentiating properties provided by optimum defoamer and wetting agent additives and the resulting improvement in aesthetic and protective properties provided by coatings. This is especially true for new resin technologies such as new waterborne alkyd resins and coatings.
References
1 Akintayo, C.O,; and Abedowale, K.O. Synthesis and Characterization of Albizia Benth Medium Oil Alkyds, Prog. Org. Coat.2004, 50 (4), 207-212.
2 Akbarinezhad, E.; Ebrahimi; Kassiriha, S.M.; Khorasani, M. Synthesis and Evaluation of Water-Reducible Acrylic-Alkyd Resins with High Hydrolytic Stability, Prog. Org. Coat. 2009, 65, 217-221.
3 Uschanov, P.; Heiskanen, N.; Mononen, P.; Maunu, S.L.; Koskimies, S. Synthesis and Characterization of Tall Oil Fatty Acids-Based Alkyd Resins and Alkyd- Acrylate Copolymers, Prog. Org. Coat.2008, 63, 92-99.
4 Dziczkowski, J.; and Soucek, M.D. A New Class of Acrylated Alkyds,J. Coat. Technol. Res.2010, 7 (5), 587-602.
5 Clark, M.D. Acrylic Modified Waterborne Alkyd Dispersions, US Patent 6,242,528, June 5, 2001.
6 Hare, C. Protective Coatings Fundamentals of Chemistry and Composition, SSPC The Society for Protective Coatings, Pittsburg, 1994, 137-148.
7 Wicks, Z.W.; Jones, F.N.; and Pappas, S.P. Organic Coatings Science and Technology,Wiley-Interscience, New York, 1999, 268-285.
8 Patton, T.C. Paint Flow and Pigment Dispersion,Wiley-Interscience, New York, 1979, 205-238.
9 Rosen, M.J. Surfactants and Interfacial Phenomenon, John Wiley and Sons, New York, 1989, 278-282.
10 Adamson, A.W. Physical Chemistry of Surfaces, Wiley and Sons, New York, 1990.
11 Schwartz, J. The Importance of Low Dynamic Surface Tension in Waterborne Coatings, J. Coating Technol. 1992, 64 (812), 65.
12 Medina, S.W. Ethoxylated Acetylenic Glycols Having Low Dynamic Surface Tension, US Patent 5,650,543, July 22, 1997.
13 Louis, C.; and Chan, S. New Additives for Waterbased Coatings: A New Class of Defoamers is Born, Eurocoat 2004.
14 Schwartz, J.; and Bogar, S. An Additives Approach to Defect Elimination in Thermoplastic Water-based Industrial Maintenance Coatings, J. Coating Technol.1995, 67, 840.
15 Reader, J.; Hegedus, C.R.; Lai, T.K.; Snow, R.A.; Tian, X.; Sun, Y.; Chaigneau, W.; Louis, C.,; Peck, M.; Sefko, J.E.; and Bennett, R. Defoamer Theory and Application in Paints and Coatings, Proceedings of the International Waterborne, High-Solids, and Powder Coatings Symposium, New Orleans, February 10- 12, 2010, Storey, R.F., Ed. University of Southern Mississippi, Department of Polymer Science.









Report Abusive Comment