This article summarizes extensive physical and optical testing of these two types of materials in several interior flat paints. It was found that most of the advantages attributed to the feldspar were in fact not borne out by the data. In general, calcium carbonate can replace feldspar with no loss and, in most cases, with an improvement in physical properties. This study found that replacing the feldspathic minerals with ground calcium carbonate resulted in overall formulation cost savings but gave comparable physical and exterior durability properties.
Introduction
Calcium carbonate is a mineral substance and may be aragonite, calcite, vaterite, chalk, limestone, marble or travertine. In the United States, commercial material is mostly calcite and is either limestone (sedimentary) or marble (metamorphic). The materials used in this study were high-whiteness, marble-based products. Marble is a metamorphic rock.
Feldspar is the mineral name given to a group of minerals distinguished by the presence of aluminum (Al) and the silica ion (SiO4) in their chemistry. This group includes aluminum silicates of soda (sodium oxide), potassium (potassium oxide) or lime (calcium oxide). Feldspar is the single most abundant mineral group on earth. These materials vary widely in chemistry, depending on local formation conditions. Some feldspathoids are silica free – e.g., nepheline syenites. Feldspars are igneous rocks. Figure 1 shows the structure of calcium carbonate and nepheline syenite. Table 1 shows the pigment properties.
Minerals with higher hardness are thought to bring improved abrasion and burnish resistance to most paint formulations, although they are more difficult to disperse and lead to faster wear on processing (disperser blades) and application equipment (pumps and spray tips).
Mineral additives with an index of refraction close to that of the resin (most latex resins are ~1.5) tend to give lower haze in clears, but they also have lower opacity and tint strength.
This research was conducted to compare opacity, abrasion resistance and burnish resistance of feldspar and ground calcium carbonate commonly used in interior flat paints. Additional work was completed in an exterior paint formulation to assess weatherability and acid rain resistance.
Experimental
Seven interior formulations were developed: a) 76% PVC; b) 53% PVC; c-f) four tint base formulations in 63% PVC; and g) a 40% PVC exterior formulation. In Formulation 1, the calcium carbonate was replaced by two different feldspars on an equal volume basis in a 76% PVC paint (Tables 1 and 2). Optical properties for all of the formulations were the same.
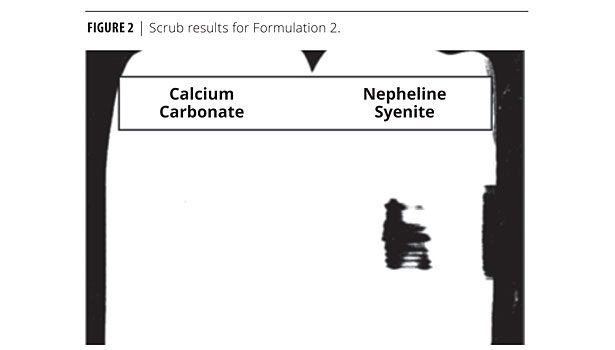
Using the same protocol as in Formulation 1, a low-VOC, 53% PVC interior flat paint was produced and tested for optical properties (Formulation 2, Tables 4 and 5). Table 5 is the optical data for Formulation 2. Scrub results are shown in Figure 2. The calcium carbonate performed much better.
Four additional formulations were produced in 65% PVC tint bases. In each formulation the calcium carbonate was replaced by nepheline syenite on an equal volume basis (Table 6). As in the first two formulations, optical properties were found to be the same regardless of mineral selection (Table 7).
Tint strength of each of the above formulations is shown in Table 8. Tint is very close regardless of mineral selection in both light and medium tint bases. The carbonate formulations show a higher whiteness in both the deep and clear bases.
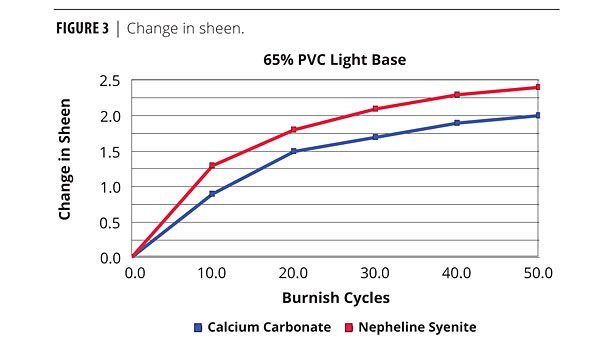
Sheen of the medium base increased after burnishing using the Imerys burnish tester (Figure 3). The carbonate shows slightly better burnish. With the other bases, the burnish of the calcium carbonate was slightly better or equal to the nepheline syenite.
Touch up was also done on these paints. The blue tinted paints were rolled on upson board. After 24 h dry, each paint was topcoated by brushing with the same paint. The touch up was evaluated by 10 people in the lab (Table 9). The numbers are the number of people that rated each sample. Overall, the carbonate showed better touch-up appearance.
Figures 4 and 5 show relative abrasion resistance (scrub resistance) measured side by side. The data clearly shows the calcium carbonate-based formulations provide superior performance, which is contrary to conventional expectations.
Formulation 7 was a commercial formulation that contained an 11-micron nepheline syenite and and a 7-micron calcium carbonate. In the first reformulation, the nepheline syenite was replaced with 12-micron calcium carbonate. The sheen was low so the ratio of the two calcium carbonates was adjusted in the second reformulation (Table 10). Scrub and staining results are shown in Figures 6-9.
Traditionally, nepheline syenite is considered to perform better in exterior paint formulations, mainly because of its perceived benefits in weathering acid rain conditions. This view was tested by simulating acid rain using the salt fog cabinet and QUV.
A 40% PVC paint formulation was tinted to a light green, and separate panels were made using a 12-micron calcium carbonate, a 1-micron coated calcium carbonate and and a 2-micron nepheline syenite.
Three panels of each were placed in the salt fog cabinet using deionized water adjusted to a pH 4 (acid rain is usually 4 to 5.5) using sulphuric acid. Three additional panels of each were placed in the QUV under UVA conditions. The last three panels were subjected to 50% acid rain and 50% QUV. Color change after exposure is reported in Figures 10a-c.
The QUV showed that the nepheline syenite had a color change early and then leveled off with the calcium carbonates with more even weathering. Given the conditions, these data are very close and do not support the expectation of large differences between these two minerals used in this mode.
Although exposure of the calcium carbonate to acid (pH 4) showed some color difference, the film itself showed no sign of degradation. The color difference found appears to be the result of mild iron oxide leaching from the system but, again, the anticipated early destruction of the carbonate-based film did not materialize, even under this aggressive environment.
Conclusions
The marble-based calcium carbonate performed similarly to the feldspathic materials in interior architectural formulations when compared at equivalent particle size and volume loadings. Opacity, abrasion resistance and burnish resistance were evaluated across a wide range of PVCs and resins.
In simulated exterior conditions, the calcium carbonate did not show the expected early failure, even after a significant time under continuous, very low pH conditions.
Based on these data, marble-based calcium carbonate can be used to replace feldspars to reduce cost while maintaining – and even improving, in some cases – physical performance.
References
1. Webmineral.com
For more information, e-mail Steve.raper@imerys.com.
This paper is based on a presentation given at the 39th annual Waterborne Symposium, February 13-17, 2012, New Orleans.




















Report Abusive Comment