Calcium Metasilicate Maintains Performance, Minimizes Cost




Lowering Raw Material Costs
In the wake of increasing prices, lowering raw material costs (RMC) is an awesome task for the coatings chemist. In order to capture the same profitability today compared to one year ago, a coatings manufacturer must charge $1.76 per gallon more just to maintain the same level of profitability based on increases from Ti02. The coatings chemist cannot sacrifice performance requirements while remaining under the ceiling of a maximum RMC. This approach has coatings manufactures around the world reformulating in ways they have not done before.
Calcium metasilicate (CaSiO3), better known as wollastonite, is a naturally occurring mineral found in several countries in the world. This unique mineral has been used in the coatings industry since the early 1960s, but is growing in ways many coatings pioneers may not have envisioned.
Former NYCO visionary Clive Hare led the charge and the use of wollastonite for a myriad of coatings, and the industry listened. Probably the most versatile paper on the use of wollastonite in coatings, written in March 1994 by Clive Hare, could be published tomorrow with only a few minor changes, as some companies referenced are no longer in business or have been integrated into another portfolio. This paper, The Evolution of Calcium Metasilicate in Paint and Coatings,is available on NYCO’s website. Hare describes how, due to the high pH of wollastonite, it is possible for untreated as well as silane-treated grades to be used as an efficient
anticorrosive pigment.
Wollastonite Properties
The physical shape of wollastonite is referred to as ‘acicular’. This unique structure allows for tighter pigment packing. Without increasing the Tg of any resin system, the network created in a coalesced film provides an additional barrier to reinforce the coating with no adverse effects.
Wollastonite has accepting Si groups, thus various treatments to this mineral are capable of coupling with wollastonite, such as functional silanes, hydrophobes, dispersing aids and specialty coatings, to further improve barrier properties, corrosion resistance or adhesion.
Research continues on utilizing wollastonite as a synergistic corrosion inhibitor. This mineral also improves coating performance in the following areas:
• Primary and/or synergistic corrosion inhibitor;
• Reduced cracking and checking;
• Improved mar/scratch/gouge resistance;
• Increased scrubability;
• Improved barrier properties;
• Thermal stability (high-temperature coatings);
• Thermal shock resistance and emissivity;
• Efficient matting agent;
• AMP 95 alternative? (buffering);
• TiO2 enhancer;
• Product consistency and quality;
• Inert, bio-soluble and non-hazardous replacement for asbestos and other man-made fibers.
Wollastonite is being used globally in all types of resin systems – 1K, 2K, water, solvent, epoxy, urethanes, acrylic and vinyls. Powder, UV-curable, coil and elastomeric coatings are all areas where wollastonite is currently used. The limitations for wollastonite are low-pH resin systems and coatings requiring a gloss above 95° at 60°.
Chemically Modified Wollastonite
Chemical modification of the engineered wollastonite pigment is performed using silane chemistry by means of a proprietary process (Figures 1a and b). Wollastonite, glass and other siliceous materials have shown themselves to be uniquely suitable for treatment with the alkoxy silanes. In the case of wollastonite, the basic nature of the mineral catalyzes the hydrolysis of the silane alkoxy groups, and, in the presence of moisture on the pigment, a silanol condensation reaction gives rise to Si-O-Si bridges across the interface of the pigment and the treatment. When the treated pigment is applied to the uniquely hospitable particle surface during the manufacturing process, it presents a more or less aligned external organic sheath bearing organofunctional groups to the environment. The environment is the binder system into which these pigments are eventually dispersed. While it is theoretically possible to specifically engineer mercapto, ureido and other groups onto the molecule, two or three systems have proven most popular as treatments on wollastonite for coating applications. 10 AS WOLLASTOCOAT®/M1250 AS presents reactive primary amine groups to the binder, 10 ES WOLLASTOCOAT/M1250 ES presents reactive epoxy groups and 10 WC WOLLASTOCOAT/M1250 WC is used in systems where vinyl type reactivity is possible.
Benefits of chemically modified wollastonite include:
• Improved homogeneity of pigment surface;
• Improved overall mechanical properties of film (adhesion/blister resistance);
• Improved corrosion resistance;
• Reduced moisture transmission through the film;
• Reduced sensitivity to acids;
• Reduced oil absorption of wollastonite particle.
Wollastonite as an Anticorrosive Pigment
Millions of dollars are lost each year because of corrosion. Much of this loss is due to the corrosion of iron, aluminum and steel, although many other metals may corrode as well. The problem with iron as well as many other metals is that the oxide formed by oxidation does not firmly adhere to the surface of the metal and flakes off easily, causing “pitting”. Extensive pitting eventually causes structural weakness and disintegration of the metal.
It has been over 25 years since the initial research calling for the use of wollastonite in a variety of coatings systems was initiated. The purpose was to examine the tendencies of epoxy silane-treated wollastonite to form synergistic pigment combinations with inhibitive pigments in water-based systems (the primary intent to be an extender pigment that aids in corrosion prevention while providing better durability was studied).
A number of functional extender pigments were compared for their contribution to the performance of anticorrosive epoxy metal primers. Because legislation restricted the use of lead- and hexavalent chromium-based corrosion inhibitors, new types of molybdate, borate and phosphate inhibitors were emerging. These had their own performance issues due to high oil absorption and lower efficiency. Examining the PVC/CPVC ratio in order to regain efficient corrosion control led to the reassessment of the choice of extender. Wollastonite has been a preferred extender pigment for improved corrosion and blistering resistance, with additional improvement derived from epoxy and amino silane chemical modification.
More recently, work was reported on the use of wollastonite chemically modified with reactive silanes, such as amino and epoxy functional types, in corrosion-resistant coatings. The role of wollastonite as a “synergist” with inhibitive pigments was described. While the mechanism is not fully known, numerous authors have described the synergistic behavior of wollastonite with the majority of primary inhibitive pigments. What is known is that when used in combination with inhibitors, wollastonite, especially the chemically modified grades, enables the inhibitive pigment to be more effective for overall corrosion protection than when the pigment is used by itself. A study focusing on waterborne epoxy primers and anticorrosive pigments reached the following conclusions regarding treated wollastonite and its performance:
• PVCs in the range of 38-45%, and a PVC/CPVC ratio of 0.6-0.9, showed the best overall coating performance when the extender package included 150 lb/100 gal of treated wollastonite and the anticorrosive pigment level was 100 lb/100 gal;
• Recoatability was excellent;
• Long-term salt spray and humidity resistance expectations were met;
• Keeping PVC at >38% inhibits blistering and flash rusting.
The past several years have seen the incorporation of wollastonite in a wide variety of corrosion-resistant coatings. While waterborne epoxies seem to prevail, the formulating trend is toward reduced VOCs for environmental compliance.
Since strontium chromate has remained an inhibitor of choice in many systems, formulators look to wollastonite to reduce the amount of strontium chromate used, without sacrificing corrosion protection. Further, zinc phosphate has been used to help offset dependence upon strontium chromate, and over the last 30 years has become the leading nontoxic inhibitive pigment. A surface-treated wollastonite has been successfully used to improve the performance of zinc phosphate in alkyds, acrylics, urethanes and epoxies.
In barrier-type formulations where corrosion resistance is achieved through means other than inhibitive pigments, surface-treated wollastonite has been shown to be effective on its own. Because the protection afforded by these types of systems is more related to controlling ionic permeability and chemical reactivity of the binder with the filler surface, a working hypothesis is that surface-treated wollastonite contributes to performance with its acicular shape and via the chemical bond occurring between the surface treatment and the binder. Some synthesis chemists have suggested the “DNA” of the resin changes, which is why some of these results are unexplainable.
Further, wollastonite’s acid solubility can be controlled by proper selection of the surface treatment chemistry. By carefully matching the chemistry to the inhibitive pigment and binder system, the contribution of wollastonite to passivation can be maintained, and the synergistic relationship can be maximized.
Improved Barrier Properties
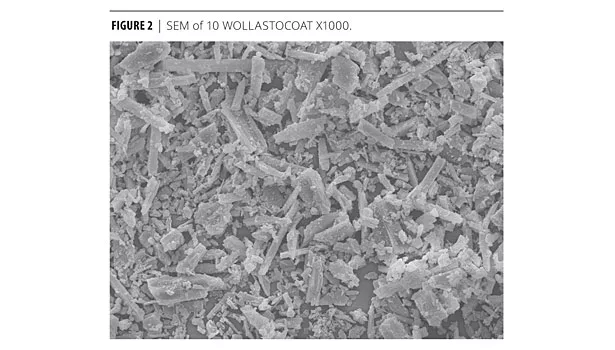
When wollastonite’s acicular shape is more closely observed, its unique make-up resembles a needle-like structure (Figure 2). In coatings, it has been found that acicularity is responsible for the following coating characteristics;
• Reinforced film cohesion and improves mechanical properties;
• Improved dimensional stability;
• Higher impact resistance and greater flexibility;
• Enhanced resistance to brittle failure and associated film degradation;
• Improved resistance to physical degradation resulting from UV radiation;
• Reduced cracking, checking, alligatoring, etc.;
• Improved durability and corrosion resistance;
• Prolonged service life of coating;
• Lower oil absorption compared to other acicular, fibrous and platy reinforcements;
• Can be used in high-solids systems to reduce VOC and lower viscosity.
These points are why increased scrubability, scratch resistance and increased flexibility of films has been shown, without increasing the Tg of the resin (Table 1). It is also important to note, it is the acicular structure that can also contribute to slight reduction of gloss.
Temperature Extremes
Creating coatings that perform in varying temperature extremes is an area wollastonite has long been used, since it melts at 1540 °C. Its use in high-temperature and even intumescent coatings is another area that needs corrosion protection – and extreme coatings are required. The heat resistance of coatings on flues, stacks, furnaces, kilns and gas heaters plays a key part in ensuring that industrial objects add and retain value. These coatings are mostly used outdoors. They must therefore be suitably resistant to corrosion and weathering. Even after many years, heat-resistant coatings for ovens, flues, stoves, stovepipes and grills are expected to keep their perfect appearance.
The selection of pigmentation is also critical to achieving maximum temperature resistance. Coatings with short-term exposures (< 1,000 h) at temperatures up to 350 °C or long-term exposure (> 1000 h) up to 225 °C can be produced with titanium dioxide and mica. Applications with short-term exposures at temperatures up to 525 °C or long-term exposure up to 250 °C often include black iron oxide. Aluminum, zinc and stainless steel are utilized in high-temperature applications (short term 650 °C, long term 350 °C). The high thermal conductivity of these metals transfers heat away from the coated substrate. In addition, as the resin oxidizes, the metal particles will fuse with the resin to form a ceramic coating with stable metallo-siloxane bonds. Further enhancements to the thermal stability of the coating can be achieved by increasing the loading of wollastonite.
Wollastonite as a Buffering Agent
Wollastonite’s high pH of 10 was identified and has been used to stabilize paints as an alternative to ammonia, TEA and potassium bicarbonate. Wollastonite in acrylic coatings showed that the zinc oxide was stabilized without a high dispersant demand. Two benefits noted were efficient raw material cost management and better package stability. Some of the overlooked benefits of wollastonite were then noted, and from there, more attributes of this mineral were studied.
• Alkaline pH stands out while requiring less ammonia and improving rust and mildew resistance, AMP partial replacement.
• High dry brightness.
• Reasonable oil absorption contributes to greater latitude for controlling PVC.
• Acicular structure demonstrates slightly lower gloss, more desirable in semigloss and flats.
• Considerably lower odor than traditionally used buffering/dispersing agents.
• Lower VOCs.
• Lower cost.
Partial TiO2 Substitute
In a recent study by an independent laboratory, it was reported that by using a 4 median micron size grade of wollastonite, and substituting 10% of a standard grade of rutile titanium dioxide, no substantial differences in delta change, LAB values or opacity were observed. Gloss was also not adversely affected in this study of an interior semi-acrylic. As Ti02 costs continue to escalate, NYCO has followed the lead of customers who expressed their appreciation that calcium metasilicate can “enhance” coatings by partially substituting titanium. Enhancing coatings is what wollastonite does. Wollastonite is not extending titanium. Tables 2 and 3 show the results of this study. We believe a perfect blend exists that can further offset the escalating costs of Ti02. A portion of this blend is wollastonite.
Conclusion
With the many options that coatings chemists have, there is an awesome responsibility to understand the virtues of a technology, the requirements of an application and then marry these two variables while offering a solution that yields a profit. There is still much to be learned about the possibilities that exist with this unique mineral, which cannot be grouped into the family of “fillers,” as this reactive pigment offers virtues far beyond that of an inert filler.n
For more information on NYCO’s wollastonite, visit www.nycominerals.com or e-mail info@nycominerals.com.
References
1. Hare, C.H. The Evolution of Calcium Metasilicate in Paint and Coatings,” Modern Paint & Coatings, Vol. 83, #12, p. 32, Nov. 1993.
2. Copeland, J.R. “Natural Calcium Metasilicate,” Chapter 1-B-d-2 p. 197, Pigment Handbook, Vol. I, 2nd Edition, Ed. Peter Lewis, Wiley, 1988.
3. Plueddemann, E.P. Progress in Organic Coatings, 11, 29 (1983).
4. Hare, C.H. Synergistic Effects of Wollastonite/ Inhibitor Concentrations in Epoxy Metal Primers, Journal of Protective Coatings and Linings, Vol. 6, #9, p. 43, Sept. 1989.
5. Revette, D.; LaRosa, S.; Phillips, G.; Palmer, J. Wollastonite: One Mineral, a World of Applications, 1982-2012.
6. Robinson, S. Wollastonite’s True Dimension, 2006.
7. Jackson, M. Guidelines to Formulation of Waterborne Epoxy Primers: An Evaluation of Anti-Corrosive Pigments, 1995.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!





