Current and Impending Developments in Silica Nanoparticle Use in UV-Curable Systems
Nanoscale colloidal silicon dioxide particles are currently widely used in UV-curable systems for surface abrasion resistance and in nanocomposites, among other applications. The use of silica nanoparticles as they are currently available is not without drawbacks, however. The natural hydrophilic surface chemistry of the silica particle has low compatibility with most UV-curable resins and crosslinkers. Monodispersed silica nanoparticles are currently either provided in a volatile solvent that must be substituted or removed, or are provided in a narrow range of monomer. This paper will review the current use of silica nanoparticles in UV-curable coatings and introduce effective approaches that have been developed for delivering silica nanoparticles to UV-curable resin formulations without the drawbacks of current solvent- or monomer-based silica nanoparticle dispersions.
Introduction
The use of monodispersed nanoparticle oxides can greatly improve the durability and performance of UV-curable coatings and composites. Colloidal silica (SiO2), one of the first commercially produced nanoparticles, is still one of the most widely used in coatings applications. Silica’s natural, glass-like hardness imparts scratch resistance to coatings while providing a clear, high-gloss finish, however the active surface chemistry of silica and the necessity to provide it in a carrier system to keep it monodispersed creates challenges for the end user. End users can overcome some of these problems through treating the surface of silica particles with silane coupling agents, however commercially pretreated silica particles, silica particles dispersed in more versatile system components, and carrier-free monodispersed silica promise to increase ease of use of silica nanoparticles in UV-curable systems.
Traditional Silica Nanoparticle Use in UV-Curable Applications

|
| Figure 1-2 Click to enlarge |
Silica nanoparticles are particles of silicon dioxide (SiO2) generally between 5 and 500 nanometers in diameter. They are usually spherical, but elongated, and other novel shapes are available (Figure 1). Silica nanoparticles generally have a density of 2.2 grams per cubic centimeter (cm3),(1) and the surface chemistry is dominated by hydroxyl groups, with between 4 and 5 per square nanometer (nm2).(1) These hydroxyl groups, attached to silicon atoms, comprise silanols. Silica is naturally anionic and always has a negative zeta potential. Its isoelectric and point of zero charge are at about pH 2.(1) Figure 2 provides a graphical representation of the silica nanoparticle structure and surface chemistry.

|
| Figure 3 Click to enlarge |
Colloidal silica nanoparticles are most commonly incorporated into coatings to provide scratch and abrasion resistance, but can also be used to enhance nanocomposite strength, modulus, control refractive index and provide a host of other surface properties including but not limited to, antiblocking and self-cleaning properties, porosity, surface roughness and hydrophilicity. Nanoscale silica is used to provide scratch and abrasion resistance to coatings because it has a natural Mohs hardness of 6-7, is optically colorless and imparts a glossy finish to coatings. Silica particles that extend slightly above the surface of the coating become the point of first contact for foreign materials, keeping them from making full contact with the resin surface. Figure 3 illustrates the orientation of silica nanoparticles in a hard coating.
Note that nanoparticles that do not protrude from the coating surface do not have an effect on scratch resistance, but may provide other benefits. Many antiscratch and antiabrasion applications in UV-curable systems require optical clarity, therefore a particle diameter below 50 nanometers, and ideally below 20 nanometers, is used. Silica solids loading for such applications is usually 10-15% for optimal scratch resistance without compromising optical clarity, coating mechanical strength and substrate adhesion. Silica nanoparticles for UV coating applications are most commonly used in commercially available monodispersions, either in organic solvents or mono-, di- or trifunctional acrylate monomers (which will be discussed later). Solvent-based dispersions are available in a wide range of solvents, including alcohols, ketones, esters and aromatics.
Drawbacks
For all the potential benefits silica nanoparticles can impart to a coating, there are drawbacks to using them in UV-curable systems.
Solvent Issues
Several significant drawbacks that present themselves when using common commercially available organic solvent-based silicas relate to the solvent itself. The first is compatibility of the solvent with the resin system. While there are many types of solvent dispersions currently available, there are still gaps where there is no commercially available silica sol dispersed in a preferred solvent.
One option that is frequently employed by end users of solvent-based silica dispersions is to redisperse the silica particles in a preferred monomer. This can be very difficult due to silica’s hydrophilic nature, and frequently risks agglomeration of the silica. This also adds another step to an end user’s process, which increases cost.
Solvent presence may also cause environmental, safety (flammability) and human health (toxicity) concerns, which low-VOC coatings seek to avoid. Even when a compatible solvent dispersion is available, the presence of solvent is not ideal. Solvents can affect rheology and cause coating defects in the cured coating. Also, solvents can offset the benefit of fast coating and curing time that UV curing can provide, as the solvent must usually be completely evaporated before curing can take place.
Effect on Coating Properties and Behavior
Like the addition of any new additive to a polymerizable formulation, the addition of nanoscale silica can affect properties, positively or negatively, outside the targeted function of the additive. Nanoscale silica can increase system viscosities, changing the rheology of the resulting formulation.(2) In UV-curable systems, the presence of silica nanoparticles can also alter the curing behavior of the system.
Researchers have found that using silica nanoparticles at levels below 10 wt% accelerated the cure reaction and cure rate in UV-curable polyester acrylate systems, but at levels above 10%, this trend was reversed. The reaction rate acceleration below 10% can possibly be explained by the silica nanoparticles behaving as an effective flow or diffusion-aid agent for the photopolymerization process, or a lengthening of the path of the UV light by partial scattering or reflection (by aggregated silica particles). Cure rate reduction at silica nanoparticle concentrations above 10% could be explained by the higher concentrations of silica nanoparticles increasing the concentration of larger aggregates, hindering absorption of UV radiation by the photoinitiator, and thus reducing the efficiency.(3)
Silica-Resin Incompatibility
As stated before, silica is naturally a hydrophilic material and, as such, can be difficult to employ in UV-curable polymer systems, which are often hydrophobic in nature. This can result in agglomeration of the silica and an overall hazy appearance in the coating, if not total failure of the formulation. Other components in the coating, such as cationic photoinitiators, can also cause agglomeration of the silica due to its anionic nature.
Conventional Remedies
Use of Silane Coupling Agents
When organic polymers are mixed with inorganic substances such as colloidal silica particles, the interphase, or interphase region, is a complex interaction of physical and chemical forces. Many mechanisms can disrupt the interphase, including water. A coupling agent creates a bond at the interphase that resists debonding, thus creating a stable bond between two otherwise poorly bonding surfaces. Silane coupling agents also enhance overall bond strength. In composites, a substantial increase in flexural strength is achievable with the appropriate silane coupling agent, as is resistance to humidity, better wetting of inorganic substrates, lower viscosities during compounding, less catalyst inhibition of thermoset composites,(4) and clearer coatings when using silane coupling agents with silica nanoparticles. Silane coupling agents are silicon-based chemicals that contain two types of reactivity (inorganic and organic) in the same molecule. The typical silane coupling agent structure is
(RO)3SiCH2CH2CH2-X,
where RO is a hydrolyzable group such as methoxy, ethoxy or acetoxy, and X is an organofunctional group such as amino, methacryloxy, epoxy, etc. They function to act as interphases between the inorganic colloidal silica and an organic resin.

|
| Table 1 Click to enlarge |
Not all silane coupling agents can be used with all silica nanoparticle dispersions. For instance, amine-type silane coupling agents such as γ-aminopropyl triethoxysilane cannot be used with silica sol, as the cationic amino group will cause gelation of the anionic colloidal silica. Care should also be taken to choose a silane coupling agent that is compatible with the solvent that silica nanoparticles are dispersed into (Table 1).
|
| Figure 4 Click to enlarge |
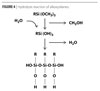
Before binding to silica nanoparticles, alkoxysilanes must undergo hydrolysis by the reaction shown in Figure 4. The water that facilitates the hydrolysis reaction is often present in small amounts in commercially available silica nanoparticle solutions, but can be added if necessary.
Once the alkoxysilane is hydrolyzed, it is ready to bond to the surface of a silica nanoparticle, as shown in Figure 5.

|
| Figure 5 Click to enlarge |
Note that the alkoxysilane-silica bond is produced via a dehydration reaction, which evolves free water that is then available to perpetuate the process of hydrolysis of other still-unreacted alkoxysilane molecules. Generally, the optimal silica nanoparticle size to use in clear, hard coatings is 10 to 12 nanometers, as larger sizes tend to cause haze. Commercially available silica nanoparticle dispersions in organic solvents are generally provided in 30% solutions. For a typical commercial 12 nanometer silica solution of 30% by weight, an amount of alkoxysilane equivalent to 1% of the total solution weight being used should be added.
The general method for preparing silica nanoparticles with silane coupling agents is to add the alkoxysilane to the silica sol and agitate at 55 °C for 3 h. This will cause approximately 20% of the –OH groups on each silica nanoparticle to be replaced by an alkoxysilane group, which is usually sufficient for promoting silica compatibility with most common monomers (Figure 6).

|
| Figure 6 Click to enlarge |
The use of silane coupling agents with silica nanoparticles not only improves the compatibility of the silica with the resin system, but also provides additional hardness to the final coating, through both covalent bonding (crosslinking) of the silica to the polymer matrix and a short-range interpenetrating network with the polymer matrix resulting from a ladder-like structure of grafted polysiloxanes.(5)
Monomer-Based Silica Sols
As previously discussed, for UV-curable systems the use of silica particles dispersed in volatile organic solvents is not preferred, so one solution has been to attempt to disperse silica nanoparticles into monomers. Examples of monomers that have had silica nanoparticles successfully dispersed in them for commercial resale include 2-hydroxyethyl methacrylate, hexanediol diacrylate (HDDA) and trimethylol propane triacrylate (TMPTA). Because of the high viscosity of multifunctional monomers, commercial silica monomer dispersions generally consist of a mono, di or trifunctional monomer.
While tri- and lower functionality may be acceptable levels of monomer functionality for some users, for others who require higher levels of functionality, this means that the monomer is acting as a reactive diluent, not a primary monomer or oligomer. Low-functionality acrylates give rise to low crosslink density and ineffective surface crosslinking, due to oxygen inhibition. This inefficient crosslinking and low crosslink density can reduce the hardness of a hard coating. Monofunctional acrylate-based silica dispersions also have high vapor pressure, causing concerns for flammability and health exposure similar to that of organic solvent-based dispersions.
Anticipated Developments
The majority of the drawbacks of using silica nanoparticles in UV-curable systems arise from the carriers into which most commercially available silica nanoparticles are dispersed, and the surface chemistry of the silica particles themselves. Therefore, logical developments to increase the ease of use of silica nanoparticles in UV systems for end users will focus on functionalization of the silanols on the particle surfaces and dispersion of silica nanoparticles into more versatile carrier systems, or elimination of a carrier system altogether.
As previously discussed, end users can increase the compatibility of solvent-dispersed silica nanoparticles with resin systems by treating the surface with trialkoxysilane coupling agents to reduce the number of exposed, active silanol groups on the particle surfaces. This process requires additional equipment, time and raw materials, increasing the expense and decreasing the ease of use for the end user. The commercial availability of silica dispersions that have already been pretreated would make silica nanoparticle dispersions much easier to use, and developments along this line are in progress.
The concept behind monomer-based silica nanoparticle dispersions is to eliminate a solvent that would not be added to a coating system if silica were not being used, but the monomers have limitations of their own, such as the low functionality of the monomers and the limited selection of monomers that are available with silica predispersed. Predispersing silica into another common system component, one that is more versatile in a variety of resin systems, is another option. Polyisocyanates with silica nanoparticles predispersed in them have recently been released commercially, and as polyisocyanates can be used in a variety of polyurethane systems, this is a very versatile delivery medium for silica nanoparticles.
The ideal form for silica nanoparticles for use in UV-curable systems would be without any carrier at all. However, when untreated silica dries, it naturally agglomerates into large, permanent clusters that cannot be redispersed as individual spherical nanoparticles. This nullifies the benefits of using nanoparticles, and results in a cloudy, high-viscosity mixture when dispersed into a resin. Therefore, in order to have commercially available monodispersed silica nanoparticles in dry form, the surface of the silica particles must be extensively modified. Research is currently underway in this endeavor.
Conclusion
Silica nanoparticles are used in UV-curable systems primarily to promote scratch and abrasion resistance, but also to provide a variety of other mechanical and surface properties. The anionic, hydrophilic surface chemistry of silica is imparted by the silanol groups that dominate the surface of silica nanoparticles, and it creates challenges for the end user of silica in UV-curable polymer systems. The conventional availability of silica nanoparticles in either organic solvents or a limited range of monomers also limits the versatility of these products in UV-curable systems. End users can overcome some of these problems through treating the surface of silica particles with silane coupling agents, however, commercially pretreated silica particles, silica particles dispersed in more versatile system components, and carrier-free monodispersed silica promise to increase ease of use of silica nanoparticles in UV-curable systems.
This paper was presented at the RadTech 2010 Technology Expo and Conference, Baltimore, MD, www.radtech.org.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!







