Use of Amino Acrylates to Reduce Photoinitiator Concentration or Increase Cure Speed in UV LED-Curable OPVs and Flexo Inks

UV LED cure is becoming more and more prevalent in several converting areas. UV-curable inkjet has used UV LED cure for pinning for many years. Generally a mercury lamp was used at the end of the press to fully cure the inkjet ink. Today, UV LED cure is being used for the entire inkjet ink converting operation. Flexo presses are being sold or retrofitted to fully use UV LED lamps. These presses include four-color process inks, plus white inks and clearcoats or overprint varnishes (OPVs). UV LED lamps are being installed on sheetfed offset presses, both in retrofits and OEM. For these types of offset presses, UV LED lamps have replaced HUV lamps as the UV curing system of choice. Other than for offset presses, these applications generally require low-viscosity formulations. All of these graphic arts applications require fast cure.
For food packaging applications, the converted product must meet, in addition to performance properties, the requirements for migration into food. The Specific Migration Limits (SMLs) of the appropriate regulation must be met. These SMLs tend to be in the parts per billion (ppb) levels.
To limit migrating species, special care should be taken in both the selection of raw materials and the manufacturing process. Raw materials should be of a certain purity, and also not contain CMRs (carcinogens, mutagens, reprotoxins). Cross contamination during processing should be avoided through proper planning, or through full GMP (good manufacturing practice). A purification step may also be needed to remove potential migrating species, and quality control (QC) may have supplementary purity/cross contamination specifications. Table 1 shows the differences between standard products, low migration (LM) products, and full GMP products that are used as raw materials in inks and coatings. The formulator must also consider these same constraints, and the convertor needs to also meet GMP requirements.

TABLE 1 » Differences between standard, low migration and GMP products.
UV LED Cure Systems
In the past, UV curing systems have typically consisted of one or two medium-pressure mercury bulbs, with broad spectral emissions, and with the wattage steadily increasing over the years from 200 to 600 watts per inch (Figure 1). These systems were generally designed to obtain the fastest cure speed possible, with productivity gains the ultimate goal. Today, curing systems, such as UV LED lamps, are being designed with other goals also in mind. Safety and environmental concerns, cure temperature, energy consumption, and maintenance schedules are all influencing the design of these new curing systems. Productivity is expected to remain the same as systems equipped with mercury lamps, but some of the newer cure systems deliver less energy to the coating, and more importantly eliminate shorter-wavelength UV. Both of these factors tend to increase the impact of oxygen inhibition on UV cure, and can negatively impact productivity.
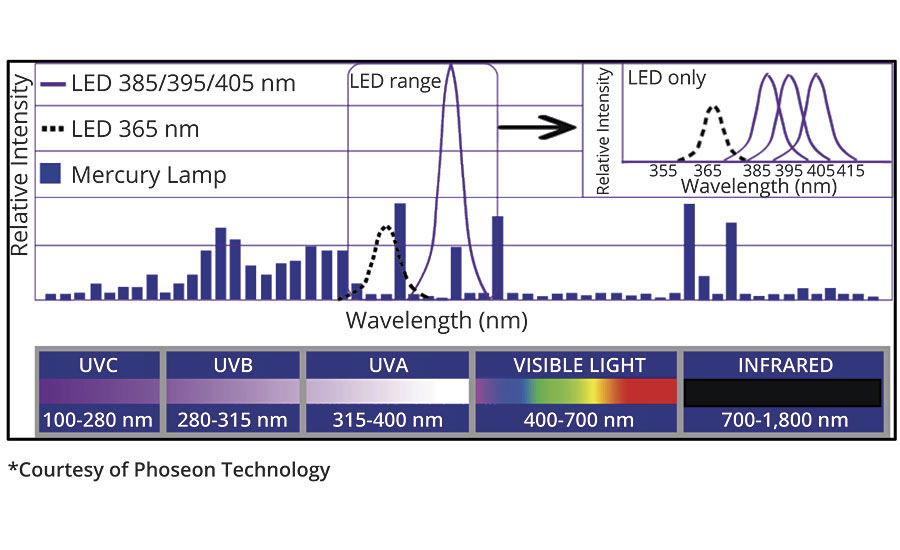
FIGURE 1 » Mercury lamp and UV LED lamp emission spectra.
Increasing the irradiance increases the concentration of free radicals, and higher concentrations of free radicals consume more oxygen. Conversely, low-energy-cure systems, with lower irradiance, result in lower concentrations of free radicals and more oxygen inhibition. The irradiance should be measured at the substrate, to determine the delivered energy, because delivered energy decreases with distance from the lamp to the substrate.
There is also a wavelength dependence on absorption of UV. UV-curable materials exhibit a higher absorbance to short wavelength energy (UVC) than to longer wavelength energy. As a result, short wavelength energy does not penetrate much beyond the surface, and longer wavelength energy (UVB and UVA) survives to penetrate deeper into the material (See Figure 1 for wavelength nomenclature). UV LED lamps do not emit UVC wavelengths, resulting in increased oxygen inhibition at the surface of the coating. However, through cure is generally very good.
Most UV LED lamps used on printing presses today emit wavelengths centered around 395 nm, in the UVA range. UV LED lamps with 385 nm and 405 nm emission bands are also used, and lamps with 365 nm emission bands are available, but only at lower intensity (Figure 1). Since their introduction, UV LED lamps have seen a steady increase in peak irradiance, from 1.1 watts/cm2 to 24 watts/cm2. Improvements in the optics of UV LEDs have also led to an increase in the irradiance that is delivered to the substrate surface.
Oxygen Inhibition and Mitigation
Generally, decreasing the viscosity of a coating increases the oxygen diffusion into the coating, and consequently decreases surface cure due to oxygen inhibition.1 However, at equal viscosity, formulations with the following characteristics will have better surface cure (less oxygen inhibition): 1) increased acrylate functionality or double bond concentration; 2) ether, amine, thiol and/or other special structural component in the backbone.2
Oxygen inhibition of surface cure is due to both quenching and scavenging reactions during free radical polymerization, as shown in Figure 2. The end result is less polymer formation and/or lower-molecular-weight polymer chains. In either case, the reaction with oxygen may give a range of results, from reduced coating properties to uncured, liquid surfaces on the coating.2
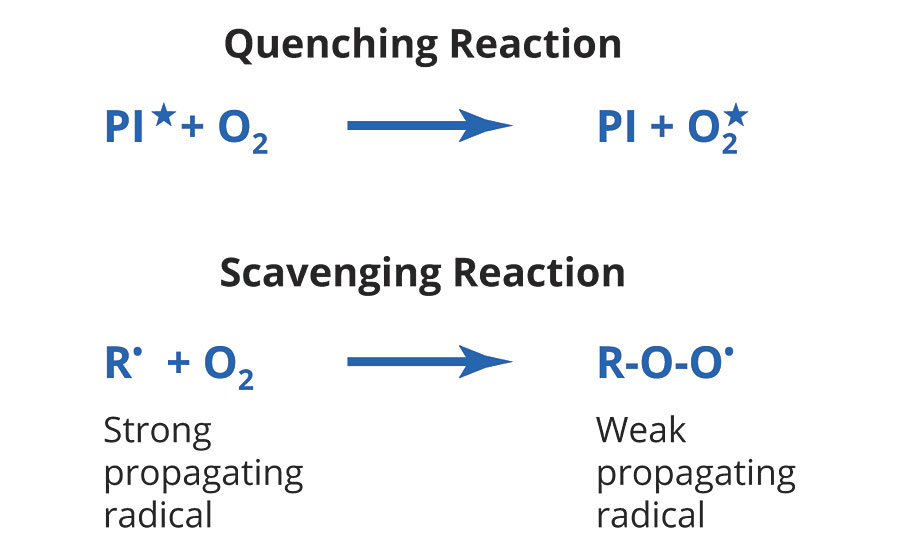
FIGURE 2 » Reactions of oxygen in UV-initiated free-radical polymerization.
There are known physical and chemical ways to reduce oxygen inhibition and improve surface cure, and these have previously been discussed.2 A summary of this topic is shown in Table 2. Also, thicker coatings suffer less from oxygen inhibition due to the bulk polymerization reaction, which increases viscosity and significantly reduces oxygen diffusion.3 Decreasing the distance from the lamp to the substrate increases the irradiance delivered to the substrate and improves surface cure. Increasing the exposure time, via slower cure speeds or multiple lamps, also generally increases the extent of cure.2
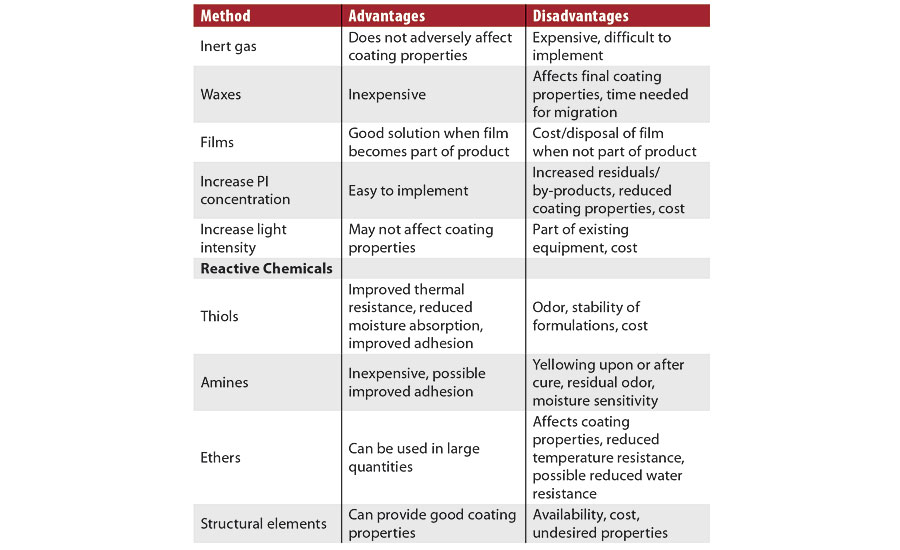
TABLE 2 » Methods to mitigate oxygen inhibition, with advantages and disadvantages.
The use of reactive chemicals is the most commonly implemented solution to mitigate oxygen inhibition. The chemicals that react with peroxy radicals contain easily abstractable hydrogen atoms. These abstractable hydrogen atoms are found in compounds containing specific structural elements, sulfur (thiols), nitrogen (amines) or oxygen (ethers). The hydrogens on the carbon atoms alpha to the sulfur, nitrogen or oxygen are those that are easily abstractable, and there are many of these in each molecule. The efficacy of these compounds, for the same equivalency, is thiols > amines > ethers. Improvements in performance are noted when the thiols, amines and polyethers are acrylated. The acrylate functionalities ensure that the materials become part of the polymer backbone after cure, and thus cannot migrate or bloom to the surface. A reduction in odor may also be obtained through acrylation.
The most commonly used solution within the reactive chemicals category is the use of amino acrylates. Overprint varnishes (OPVs), flexo inks, inkjet inks and screen inks all use this technology. In some cases, added benefits such as improved adhesion and increased color strength are obtained. Increased yellowing on cure is seen with some amino acrylates, and this can be a problem for OPVs and white inks. Also, litho inks can have issues with the use of some amino acrylates, as they can react with acidic fountain solutions, and can also disrupt the ink-water balance.
For amino acrylates to fully function, a Type II photoinitiator should be utilized. Figure 3 shows the photoabstraction reactions of a Type II photoinitiator (benzophenone) with an amine. Other Type II photoinitiators are thioxanthones and anthraquinones. In most formulations, combinations of photoinitiators are used to ensure both surface and through cure. Amino acrylates can vary in reactivity, depending on their amine content and double bond concentration. The number of abstractable hydrogens and their accessability (steric hindrance) also affect the reactivity of the amino acrylate. The concentrations of both the photoinitiator(s) and the amino acrylates should be optimized for each formulation. In many cases, the use of amino acrylates can reduce the amount of photoinitiator(s) without impacting cure speed.
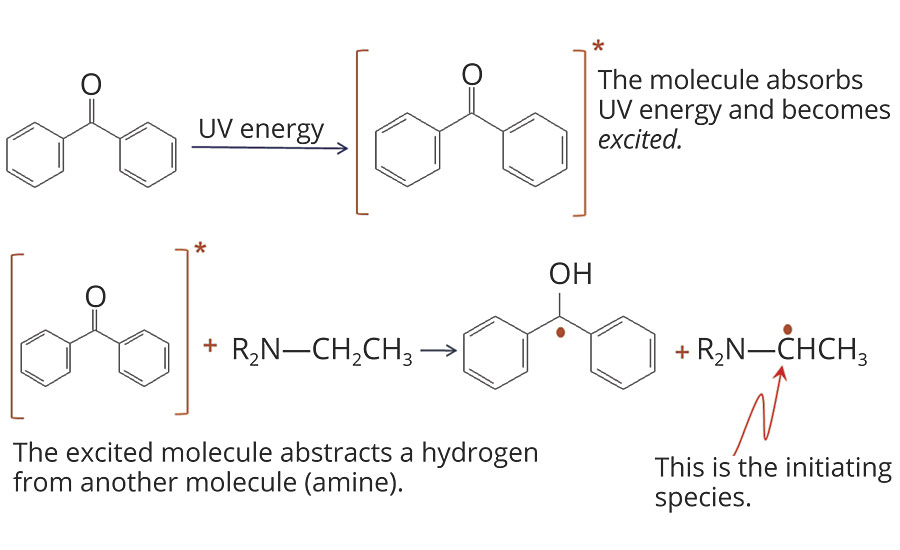
FIGURE 3 » Photoabstraction reactions with benzophenone and amine.
In this article, several amino acrylates will be evaluated for their capability to reduce oxygen inhibition and increase surface cure in UV LED-curable overprint varnishes. Data on cure speed, as well as other properties will be presented. In flexo inks, the impact of amino acrylate content on cure speed and/or photoinitiator levels will be assessed and discussed.
Material Descriptions
The three amino acrylates evaluated in these studies are shown in Table 3. AA1 is a very low-viscosity, high-amino-content material with no acrylate functionality, and typical use levels of 5-15%. (Although AA1 does not contain acrylate functionality, it will still be designated as an amino acrylate in this article.) AA2 is a high-viscosity diacrylate with the lowest amino content. The typical use levels of AA2 are 8-15%. AA3 is a medium-viscosity diacrylate with medium amino content and typical use levels of 5-20%. AA3 is also designed to be a low-migration material.
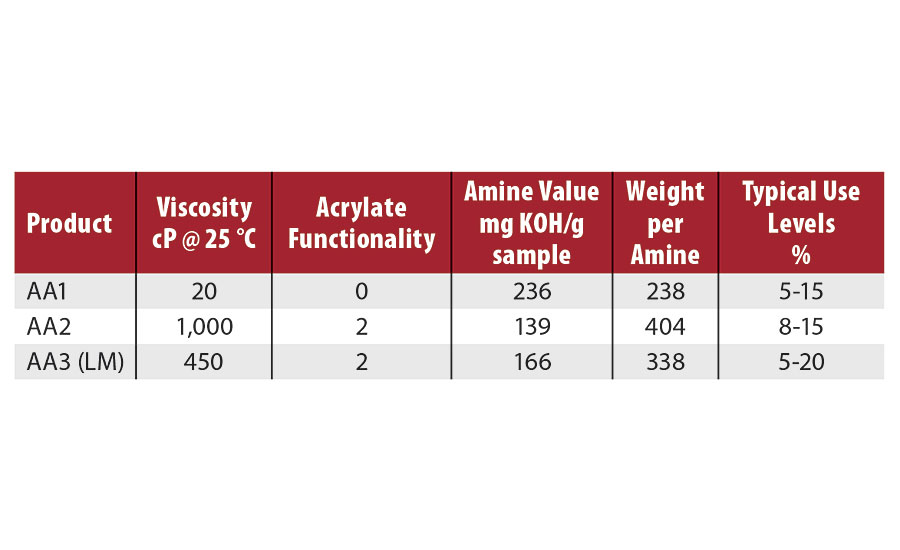
TABLE 3 » Description of amino acrylates.
The amino acrylates were used in several OPV and flexo ink formulations that utilized some of the oligomers shown in Table 4. EA1 is bisphenol A epoxy acrylate diluted in 40% trimethylolpropane triacrylate (TMPTA). This type of oligomer is traditionally used in nonfood packaging OPVs. PEA1 was designed as a BPA-free drop-in replacement for EA1, useful for OPVs for food packaging. PEA2 and EA2 are both BPA free, and may also be useful in food packaging OPV applications. PEA3 is a low-migration, six-functional material used to maintain viscosity in some formulations. PEA4 is a good pigment wetting oligomer used to grind pigments for flexo inks. Ethoxylated trimethylolpropane triacrylate (TMPEOTA) and/or propoxylated neopentylglycol diacrylate (NPG(PO)2DA) were also used as diluents in the OPV and flexo ink formulations. The high-functionality pentaerythritol tri/tetra acrylate (PETIA) was used to increase cure speed in flexo inks.
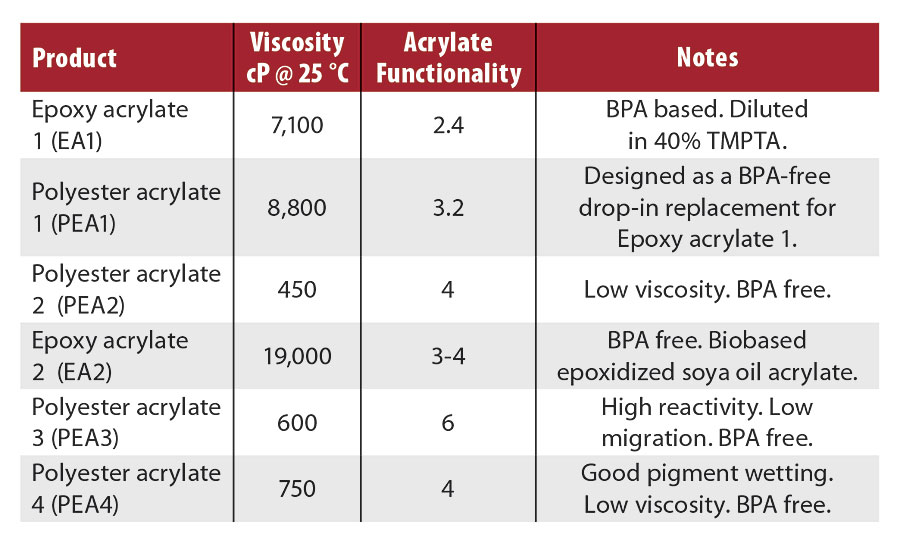
TABLE 4 » Oligomer descriptions.
Photoinitiators (PI) that absorb in the UVA range are required for UV LED cure. A liquid phosphine oxide type (TPO-L) was used as the main PI in these studies. A thioxanthone type PI sensitizer (DETX) was also evaluated, along with a substituted phosphine oxide (SPO) that requires an amine to be effective.
Photobleaching is particularly pronounced with phosphine oxide type photoinitiators that absorb in the long wavelength (~ 400 nm) region of the spectrum. These photoinitiators are yellow in color before cure. With photobleaching, the absorbance (and yellow color) decreases with exposure time to light of the proper wavelength. This occurs because the absorption characteristics of the photolysis products (from the cleavage reaction) are different from that of the original photoinitiator molecule. Photobleaching enhances optical penetration and lowers the final color of the cured coating or ink. Moreover, this photobleaching continues with time after cure, and the final color is further reduced after 24 to 36 hrs. Thioxanthone type photoinitiators do not photobleach.
Test Conditions
The OPVs were formulated by mixing all of the components together with a speed mixer. The OPVs were then applied to white Leneta charts with a #2 wire wound bar, resulting in a 5-micron-thick film. Cure was with an air cooled 16 watt/cm2 395 nm UV LED lamp from Phoseon, at a cure distance of 1 cm. Cure energy was measured with an EIT 365/40W radiometer. Cure speed was determined as the fastest line speed to give a mar-free surface as tested with a wooden tongue blade. The yellowing index was reported as the b* value measured with a BYK Spectroguide Sphere. Yellowing was measured immediately after cure and 24 or 36 hrs after cure.
The flexo ink pigment pastes were prepared on a three roll mill using PEA4 and a dispersing agent to grind the pigment. Pigment was ground until a reading of 8 on the Hegman gauge was obtained. This corresponds to 0 mils or 0 microns particle size. The pigment paste was then letdown with a blend of oligomers, monomers, amino acrylates and photoinitiators. The letdown blend was prepared with a speed mixer until homogeneous. The flexo inks were then applied to white Leneta charts or filmic substrates with a flexo proofer to obtain various optical densities. Cure was with either an air-cooled 16 watt/cm2, 395 nm UV LED lamp or an air-cooled 8 watt/cm2, 365 nm UV LED lamp, both from Phoseon, at a cure distance of 1 cm. Cure speed was determined as the fastest line speed to give a mar-free surface as tested with a wooden tongue blade or a thumb twist. Optical density was measured with an X-Rite 500 series spectrodensitometer or a Gretag MacBeth Spectro Eye portable spectrophotometer. Adhesion was tested via tape pull with TESA 4104 tape.
Viscosities at different shear rates were measured with an Anton Paar Physics MCR 101 Rheometer. The shortness index (SI) was calculated by dividing the low shear viscosity at 0.1 s-1 by the high shear viscosity at 2500 s-1.
UV LED-Curable OPV Formulations
Table 5 shows the OPV formulations that were tested in order to optimize the photoinitiator levels and the acrylated amine levels. (There are viscosity effects with UV LED cure, so the formulations (except 184) were designed to maintain similar viscosities.) The main photoinitiator, TPO-L, could be reduced from 13 to 5% through the use of 15% AA3 and 1% co-initiator, SPO. The cure speed was maintained at 70 fpm in the optimized formulation #194. Without the use of amino acrylates, the cure speed of formulation #194 was <15 fpm. This optimized PI package will be used in further studies.
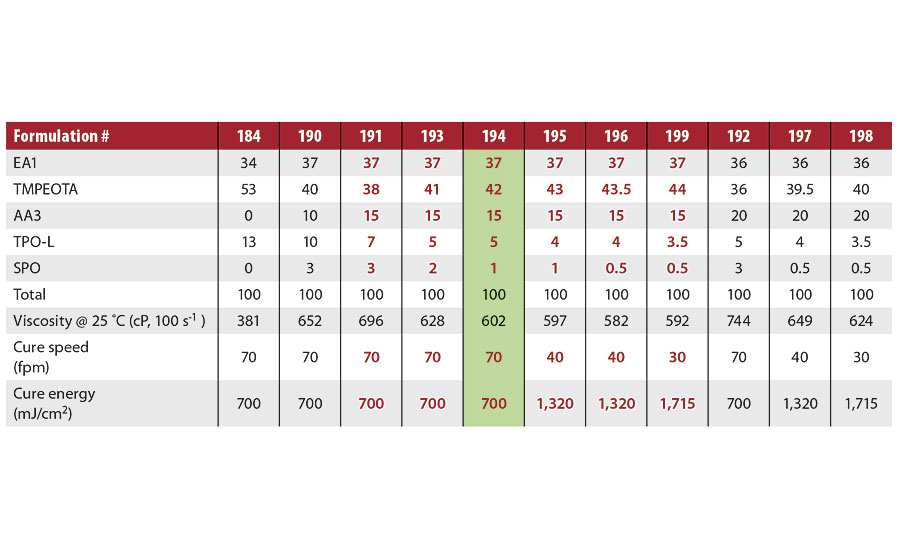
TABLE 5 » Photoinitiator package optimization.
The co-initiator, SPO, was chosen based on earlier work that evaluated a photoinitiator package containing 10% TPO-L and 3% of either DETX or SPO. The cure speeds of both PI packages were the same with the various oligomers. However, the yellowing index was dramatically different for formulations containing either DETX or SPO. The DETX-based formulations resulted in yellowing indices in the range of 9 to 17. The SPO was much less yellow, with yellowing indices of 5 to 8. The DETX is also on the Nestle Exclusion List, and should not be used in coatings or inks intended for food packaging.
The yellowing indices of the formulations shown in Table 5 were all around 4 (initial), and were reduced about 0.1 units after 24 hrs. (These yellowing indices were for OPV formulations that were stored for one week and then cured.)
Using the optimized levels of photoinitiators and acrylated amine, cure speeds were assessed for multiple oligomer types using the three different acrylated amines. Table 6 shows these data. Cure speeds varied with oligomer type. The bisphenol A based epoxy acrylate (EA1) had the fastest cure speeds regardless of which acrylated amine was used. Bisphenol A-based epoxy acrylates are known for their fast cure speeds with mercury lamps, and this fast reactivity remains with UV LED lamps. PEA1, the drop-in replacement for EA1, was the next fastest, followed by the epoxidized soya oil acrylate (EA2) and the PEA 2/PEA3 blend. For all of the oligomer types, the use of AA3 provided the fastest cure speed. Not shown are data for the Table 6 formulations that did not contain acrylated amine. These formulations did not cure at even the slowest conveyor speed of 15 fpm.
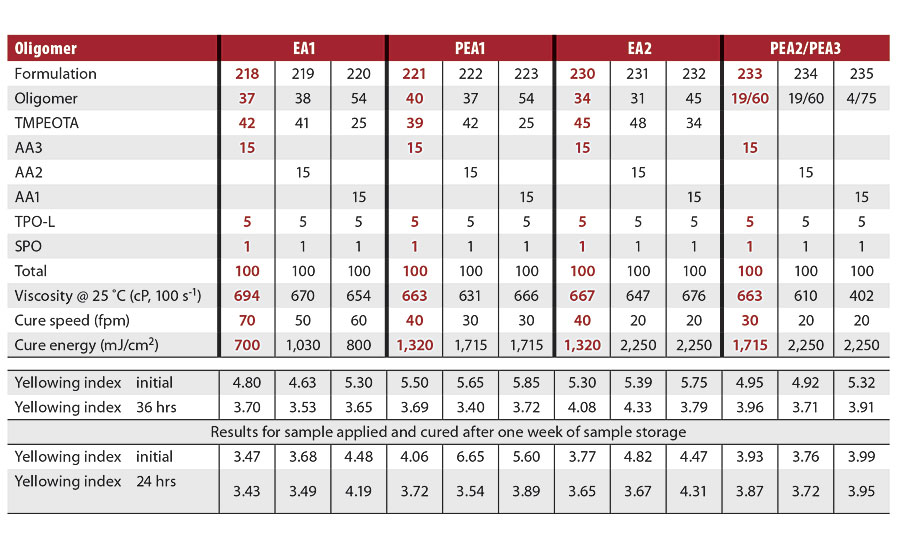
TABLE 6 » Cure speed and yellowing index of OPV formulations with various oligomers and acrylated amines.
If a food packaging OPV is required, only the formulations that use AA3, and do not use EA1, are BPA-free and based on materials that could be appropriate for this application.
Table 6 also reports several yellowing indices. The yellowing index was measured on formulations that were freshly prepared and cured the same day, and on formulations that were stored for one week and then cured. Additionally, the yellowing index was measured immediately after cure, and 24 or 36 hrs after cure. In all cases, photobleaching was observed as a reduction in the yellowing index 24 or 36 hrs after cure. The final yellowing index ranged from 3.4 to 4.3 for all formulations.
Surprisingly, the formulations that were stored for one week and then cured gave initial yellowing indices that were generally one unit lower than the formulations that were prepared and cured the same day. There was not much difference in the yellowing indices measured after 24 or 36 hrs, however.
UV LED-Curable OPV Conclusions
For UV LED-curable OPV formulations, a low-yellowing PI package has been identified. The main photoinitiator, TPO‑L, could be reduced from 13 to 5% without impacting cure speed, through the use of 15% acrylated amine (AA3) and 1% co-initiator (SPO). The cure speeds of three acrylated amines were compared. AA3 was faster than AA1 or AA2. The AA3 is also the only acrylated amine designed for low-migration applications such as food packaging.
UV LED-Curable Flexo Ink Formulations
Table 7 shows cyan and magenta flexo ink formulations that were cured with the 395 nm, 16 watt/cm2 UV LED lamp. The pigment base consisted of 45% pigment, 55% PEA4 and 3.5% dispersing agent. The PI blend (TPO-L/DETX 50/50) content remained constant at 5.4% in these experiments, and optical densities were also essentially the same for each color ink. At AA3 levels of 9.1%, cure could not be achieved with three passes at 15 fpm for either the cyan or the magenta ink. Increasing the content of AA3 to 15% gave cure speeds of 50 fpm for both color inks. Increasing the AA3 content helps to mitigate oxygen inhibition, providing for the faster cure.
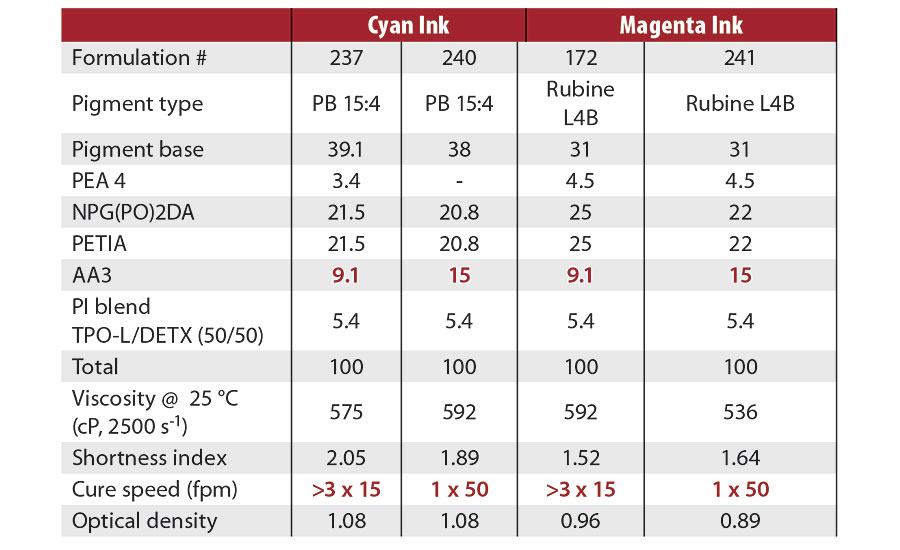
TABLE 7 » Cure speeds of cyan and magenta flexo inks with various AA3 contents (395 nm, 16 watt/cm2 UV LED lamp).
Similar results were obtained for cure with the 365 nm, 8 watt/cm2 UV LED lamp. Increasing the AA3 level from 9.2% to 15.3%, while holding the PI level around 4.5%, increased the cure speed from 16 fpm to 57 fpm. This is more than a threefold increase in cure speed.
Table 8 shows the differences in cure speed for cyan inks with different optical densities. The optical density of the inks is related to their thickness. The ink with an optical density of 2.27 (Ink A) was approximately 4 microns thick. (The thicknesses of the other inks are unknown.) Increasing the optical density or thickness of the ink increased the cure speed, as clearly shown for inks B-D. Thicker inks and coatings show less oxygen inhibition for reasons discussed earlier, and thus have better surface cure. For the formulations shown in Table 8, there appeared to be a minimum ink thickness for good surface cure, correlating to an ink optical density somewhere between 1.4 and 1.9. However, if the acrylated amine (AA3) concentration was increased from 9.1 to 15% (see Table 8 Formulation B, and Table 7 Formulation 240), the cure speed of the ink with an optical density of 1.08 increased from no cure with three passes at 15 fpm to full cure with one pass at 50 fpm. This finding is very important as inks are generally printed to target a certain optical density.
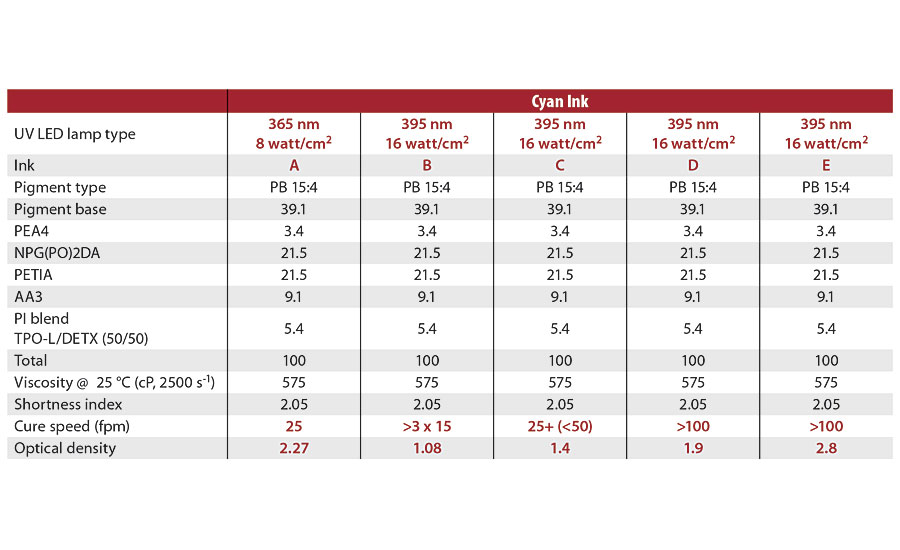
TABLE 8 » Cure speed differences for cyan inks with different optical densities.
Table 8 also shows the differences in cure speed for cyan inks cured with either the 365 nm lamp or the 395 nm lamp. For the same optical density ink, a cure speed of 100 fpm was obtained with the 395 nm, 16 watt/cm2 UV LED lamp versus only 25 fpm with the 365 nm, 8 watt/cm2 UV LED lamp.
These data demonstrate the need to optimize the flexo ink formulation for thickness and/or optical density of the ink, type of UV LED lamp and desired cure speed.
Adhesion to several filmic substrates was tested using the formulation shown in Table 8 cured with the 365 nm UV LED lamp (Ink A). Perfect adhesion was obtained on polypropylene (PP), polyethylene (PE), polyvinyl chloride (PVC) and polyethylene terephthalate (PET). These adhesion results indicate good through cure of the flexo inks.
UV LED-Curable Flexo Ink Conclusions
Acrylated amine (AA3) can be used in flexo ink formulations to increase cure speed while maintaining the PI concentration. With a 365 nm UV LED lamp, the cure speed can be more than tripled if the AA3 content is increased 1.67 times. For a 395 nm UV LED lamp, formulations with the low levels of AA3 did not cure at all, and increasing the AA3 concentration 1.67 times gave cure speeds of 50 fpm.
Surface cure is very dependent on the thickness of the flexo inks. However, increasing the acrylated amine content can provide for cure of low-thickness flexo inks. At the same optical density, cure of flexo inks with the 395 nm UV LED lamp was faster than cure with 365 nm UV LED lamp.
Conclusions
Acrylated amines can be used to either increase UV LED cure speed at existing PI concentrations, or maintain UV LED cure speed at reduced PI concentrations. AA3 was the most effective acrylated amine in the OPVs and flexo inks that were studied.
For UV LED-curable OPV formulations, a low-yellowing PI package was identified that should be acceptable for food packaging applications. The low migration AA3 is part of this formulation.
UV LED cure of flexo inks is very dependent on the thickness of the ink. Increased acrylated amine (AA3) concentration allows for full cure of flexo inks with low thickness. Cure of flexo inks with the 395 nm, 16 watt/cm2 UV LED lamp was faster than cure with the 365 nm, 8 watt/cm2 UV LED lamp. Good adhesion to several filmic substrates was obtained for the flexo inks cured with the 365 nm UV LED lamps, indicating good through cure.
References
1 UV Coatings: Basics, Recent Developments and New Applications, pp179-184. Reinhold Schwalm. Elsevier 2007.
2 Arceneaux, J.A. Mitigation of Oxygen Inhibition in UV-LED, UVA and Low-Intensity UV Cure. UV+EB Technology, Vol. 1 No. 3, pp 48-56.
3 Fouassier, J.P.; Rabek, J.F. Radiation Curing in Polymer Science and Technology, Volume III, pp 33-64. Elsevier Science Publishers LTD 1993.
Acknowledgements
The experimental work was carried out and reported on by my co-authors and colleagues: Celia Buono, Kevin Poelmans and Carmen Van Vaerenbergh.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!




