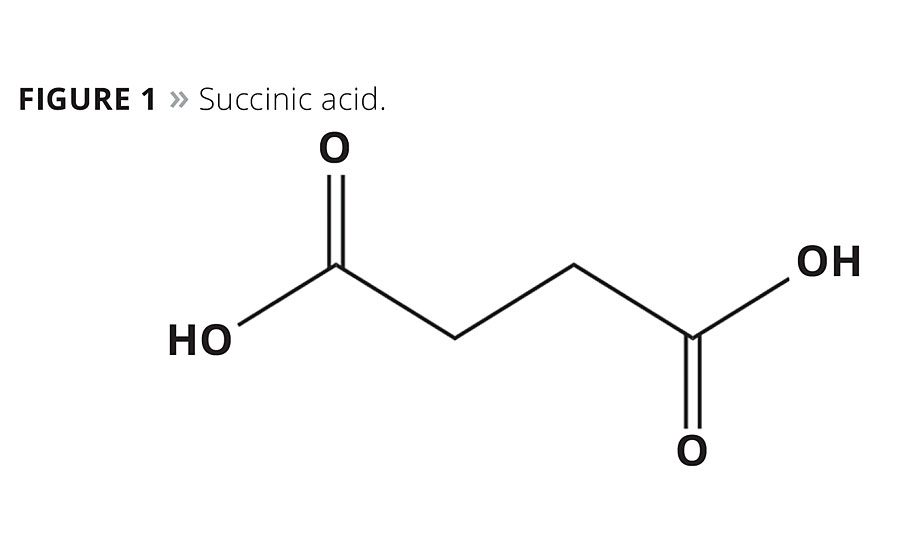
The finite availability of petro-chemical resources, along with their environmental impact and economic volatility, have long been known to the petro-chemical industry and are more frequently viewed by society and the consumer as unsustainable. Society’s awareness of petro-chemicals’ ecological footprint is growing, along with knowledge that the unchecked use of these materials will result in depletion of the earth’s natural resources and further environmental damage for future generations. This awareness has initiated a movement within the textile, materials and coating industries to produce products in a more sustainable manner. Stahl, a leader in process chemicals for leather and other substrates, recognized this trend early on and developed a wide variety of water-based products using technologies such as high-performance polyurethanes to develop innovative products that meet or exceed customers’ performance expectations and reduce environmental impact. Together with partners such as BioAmber, Stahl is developing new products where the petroleum-based polyols are either fully or partially replaced by renewable countertypes. Using Bio Amber’s biobased succinic acid (SA) (Figure 1) as a key building block of polyester polyols (PEPs), an important group of materials can be formed that enable the creation of polyurethanes (PUs) and polyurethane dispersions (PUDs) for coatings with excellent performance. These can be produced in a sustainable fashion, reducing carbon emissions and energy consumption.
As a platform chemical, biobased succinic acid provides researchers and product developers a sustainable chemical building block to enable innovative development of high-performance products useful in a wide range of applications ranging from personal care products, to non-phthalate plasticizers, to polymeric derivatives used in urethanes, polyesters and alkyd resin technologies.
Over the last few years, BioAmber and partners, such as Stahl, have invested resources to investigate the structure-property relationship of biobased succinic acid in polyester polyols for urethanes, polyester thermoplastics and polyester alkyd resins. These efforts have resulted in broad application understanding in a variety of areas such as polyurethane coatings and resins. New products utilizing biobased SA as a key component of the resin formulation are continuing to emerge and enhance both performance and sustainability of the final formulations. Many of these application studies have been published1-4, 7 and have helped catalyze the market adoption of biobased SA for use in the PU and CASE markets. In addition, starting later in 2015, BioAmber’s Sarnia production facility will enable the availability of consistent, high-quality biobased succinic acid for these and other markets.5
Results and Discussion
In PU applications, succinic acid is modified with dialcohols (glycols) to produce polyester polyols, as shown in Figure 2. The nature of the C4 diacid combined with a glycol or a mixture of glycols results in polyester polyols with a diverse range of properties. In contrast to the more familiar petro-based adipate polyols, biobased succinates provide a range of properties that can replace or compliment the petro-based adipates. Careful control of the glycol to acid stoichiometry enables very precise control of the degree of polymerization (n), which is typically reported as the OH number (the hydroxyl, or OH number, is reported as mg-KOH/g polyol). This relationship is well-known and will not be covered in detail in this article. However, an excellent overview of polyurethanes and the key building blocks used to develop materials with well-defined physical properties can be found in a number of published resources.6
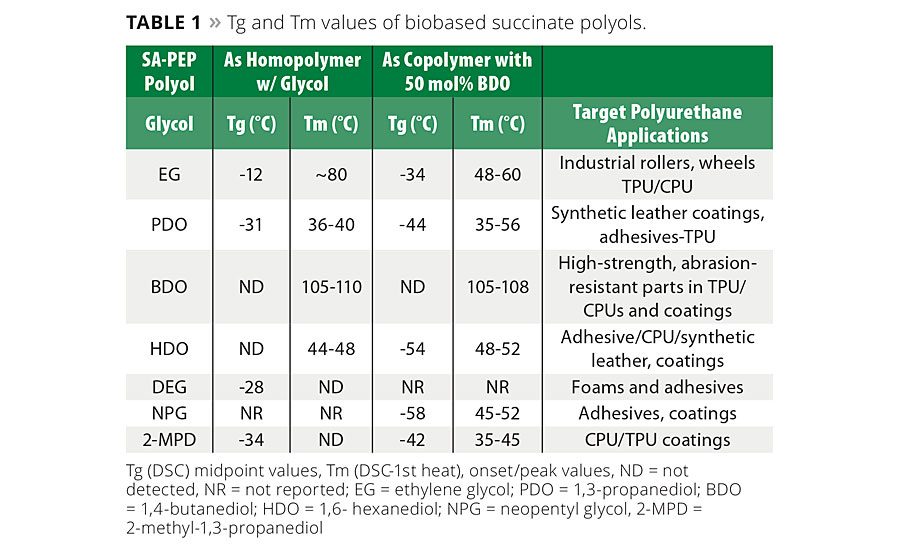
Table 1 provides a general summary of how different glycols and mixtures of glycols influence the glass transition temperature (Tg) and melting point (Tm) of succinate polyols. In general, when the C4 succinic acid is used instead of the C6 adipic acid in polyester polyols and subsequently in polyurethanes, the influence of the shorter diacid translates into performance benefits such as higher mechanical strength, higher modulus and hardness, improved abrasion and better solvent resistance.
Some potential trade-offs compared to adipates are that the SA-PEP will tend to have a higher Tg and viscosity. Typically, SA-PEPs prepared using even-numbered carbon glycols with SA are solids at 25 °C, whereas odd-numbered carbon glycols or mixed glycol systems produced viscous liquids at room temperature (RT) (see Table 1 for definitions of abbreviations). However, as has been noted previously, SA-PEP with at least 50 mol% of BDO solidifies at room temperature, whereas the corresponding adipic acid (AA)-based PEPs remain viscous liquids at RT. For example, SA-NPG/BDO is a solid at room temperature but AA-NPG/BDO is a liquid at RT. Anomalously, SA-PDO will also slowly crystallize at room temperature and has a melting point between 35 - 43 °C. Figure 3 shows graphically some of the trends in Tg as a function of glycol structures in SA-BDO/X PEPs. Additional Tg and Tm data are available in Reference 2.
In our second in-depth study in PUDs,1,4 we wanted to further our understanding of the structure-property relationship of succinate polyester polyols in an attempt to gain additional performance understanding of PU coatings derived from different succinic polyester polyols -especially relative to glycol structure. Tables 2 and 3 show the properties of the polyols synthesized in this study, some of which were made into PUD and PU coatings for further evaluation.
Characteristics of PUDs and Coatings Made with PEPs
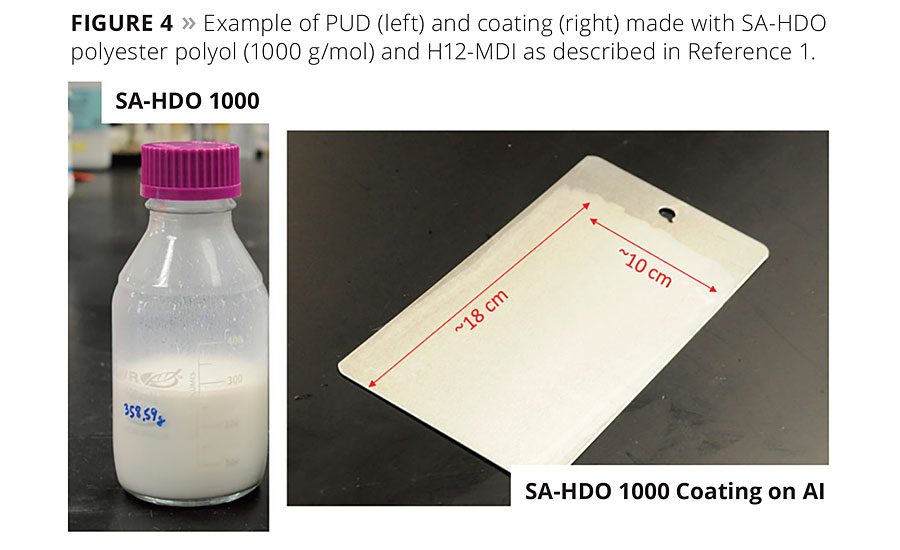
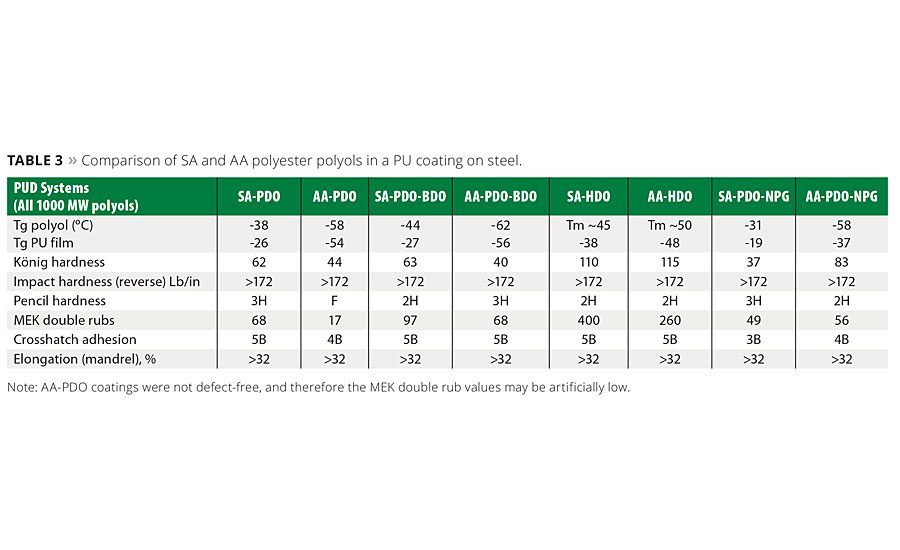
PUDs were prepared from the SA-PEPs shown in Table 2 using either the NMP co-solvent process or by the acetone process.1,4 The use of the acetone process produces an NMP-free PUD dispersion and is highly desirable from an environmental perspective. A total of six PUD formulations were prepared, coated on steel substrates and evaluated by well-known coating characterization techniques.1,4 A typical example of a PUD and the metal-coated articles is shown in Figure 4, and a summary of the properties for these coatings in this study is shown in Table 3. These generalized coatings were compared to similar PU coatings made from AA-PEPs. However, it should be noted that since this was a generalized study to evaluate side-by-side the impact of the SA or AA PEPs’ molecular structure on the physical properties of the polyurethane coatings, the formulations used in the PUD study contained no crosslinkers, adhesion promoters or surfactants and were unoptimized for any particular application.
The coated property data in Table 3 suggest the polyols made with linear SA-HDO systems (even-numbered carbon glycol) generated coatings with the highest König hardness in the series, whereas the PU coatings made with odd-numbered glycols or mixed/branched glycols had much lower hardness values. It is important to note that although the hardness values did drop with the “softer,” more-flexible polyols, that other key properties were consistent with the performance attributes associated with the SA-polyols. That is, the PU coating on the metal substrate exhibited good adhesion, solvent resistance and elongation. As evidenced by reverse impact hardness and mandrel bend tests, all of the PUDs formed very flexible coatings. In the reverse impact test, all coatings scored above the upper measurement limit of the instrument, and in the conical mandrel bend test, no signs of cracks were observed at the smallest mandrel diameter for all coatings. Table 3 and Figure 5 show how some of the mechanical properties of PU coatings based on succinates compared to the properties of similar coatings made with adipates. It was observed in this study that the PU coatings comprised of biobased SA polyester polyol generally exhibit equal or better coating hardness and better solvent resistance than the AA polyols. This was clearly noted in the SA-HDO, SA-PDO/BDO and SA-PDO systems as the MEK solvent-abrasion resistance is superior to corresponding adipates.
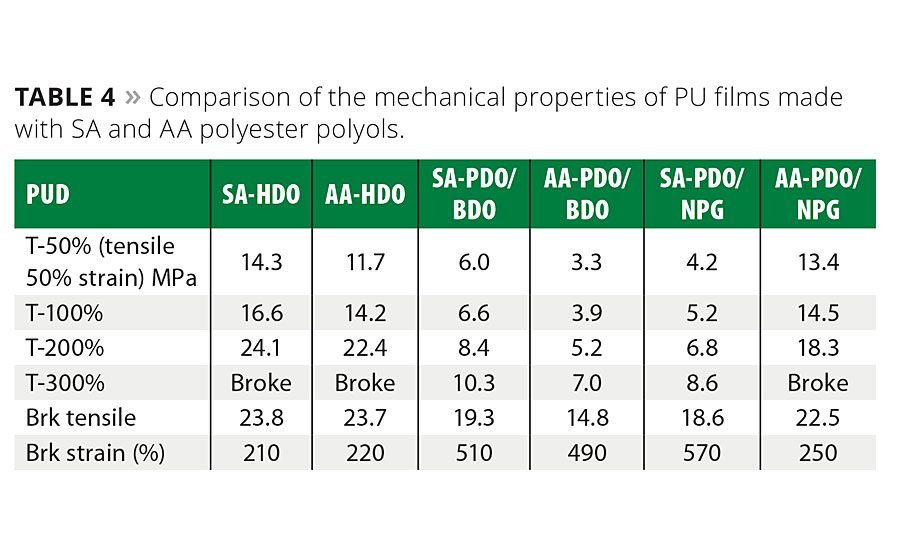
Mechanical Properties of PU Films Made from PUDs
The mechanical properties of the PU films were evaluated by tensile testing using the method described in Reference 1. Representative stress-strain data and the average moduli values from stress-strain tests are summarized in Table 4. Additional mechanical property data can be found in References 1 and 4.
Uralkyd Introduction
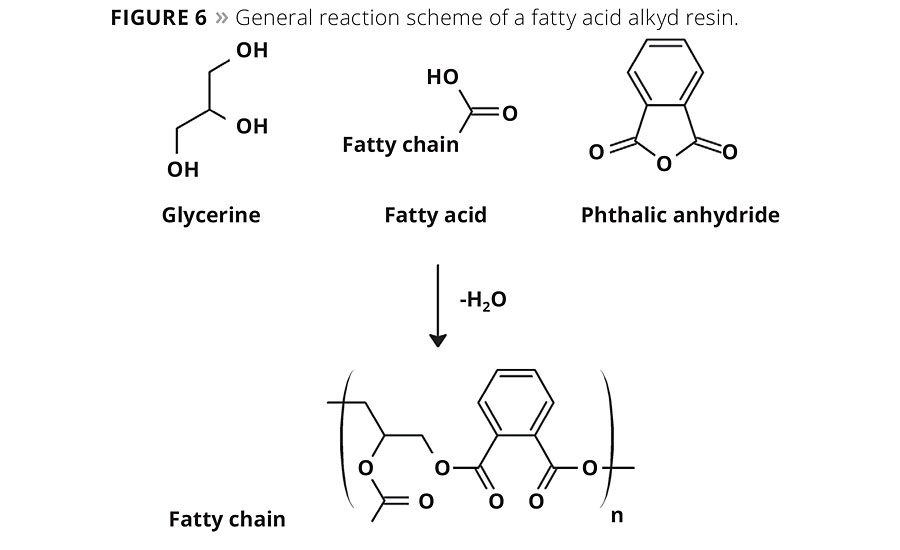
Alkyd resins have been known in the coatings industry for years and have been commercially available since the 1930s. As a class of polyester coating resins, alkyds are one of the most important binders in the paint industry and are likely to remain important for the foreseeable future. Alkyd resins are short, branched polyester chains formed by the condensation reaction between polyols, such as glycerine, trimethylolpropane and diacids or anhydrides such as phthalic anhydride, maleic anhydride and unsaturated fatty acids. A generalized reaction scheme of an alkyd is shown in Figure 6. Traditional one-part (1K) alkyd formulations are popular for wood coating applications due to their ease of use, excellent aesthetics, long-term durability and favorable economics. This makes the 1K alkyds one of the most common products for the DIY market. Even though alkyds contain a significant amount of renewable biobased carbon, the use of white spirits or aromatic solvents detracts from this reduced carbon footprint and makes them less environmentally and worker friendly.
It is important to note that the drying speed and durability of an alkyd coating and the solution viscosity are directly related to the molecular weight of the alkyd resin. High-molecular-weight alkyds tend to have a faster drying speed and better durability, but high-solid formulations have higher solution viscosities and, therefore, need more solvent to obtain a workable coating viscosity. Therefore it would not be unusual for a premium performance alkyd formulation to have as much as 70% solvent in order to balance the end physical properties with an acceptable processing viscosity.
Uralkyds Based on Succinic Acid and Fatty Acids
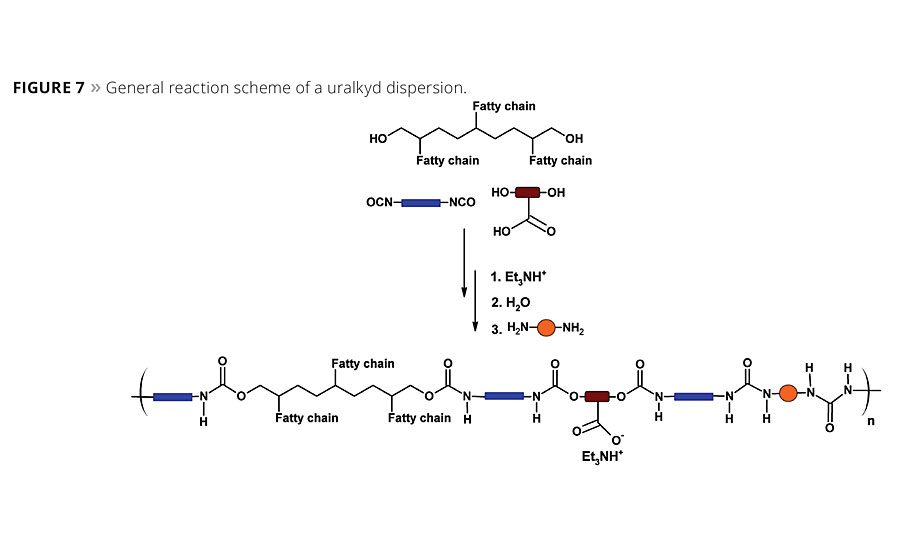
The development of uralkyds merges the formulation flexibility of water-based PUD technologies with alkyds to address the performance-processing trade-offs and enables high-performance, water-based, high-solids formulations.7,8 In waterborne polyurethane chemistry the viscosity of the emulsion is not related to the polymer molecular weight since the emulsified particles do not significantly impact solution viscosity. Thus, by combining PUD technology with alkyds it is possible to obtain a high-molecular-weight uralkyd with excellent processing viscosity, fast drying and good durability.9 Schematically, the synthesis of uralkyds is shown in Figure 7 and, as can be noted, it is similar to PUD synthesis in that a polyester polyol is mixed with or replaced by a fatty acid polyol and reacted with a diisocyanate, chain extender and a dispersing acid, typically 1,3-dihydroxy-2-propanoic acid, (DMPA).
High-performance wood coatings are a highly desirable application area and one that combines material science and innovation to meet the critical in-application performance requirements such as excellent optics, scratch, stain, solvent resistance and color fastness. In this application area, the uralkyds excel since this material platform merges many of the benefits of alkyds and polyurethanes to meet and exceed customer performance and value expectations.
The original Stahl products Picassian®HY-614 (NEP co-solvent based) and Picassian HY-460 (no co-solvent) were developed using polyesters made from a mixture of acids based on adipic (AA), glutaric (GA) and succinic acids (SA). This acid mixture, known as AGS dibasic acids, tends to have inconsistent ratios of these three acids, leading to downstream performance and regulatory challenges. Therefore Stahl decided to reformulate with polyesters based either on petro-adipic acid or biobased succinic acid. With the greater availability and quality of biobased succinic acid, and a commitment to green solutions and innovation, it was a natural fit that this reformulation opportunity be used to develop a high-performance wood coating with a greater content of sustainable, green materials. However, the biobased succinic acid derivatives had to deliver performance and value in use before these products could be converted to the biobased building block.
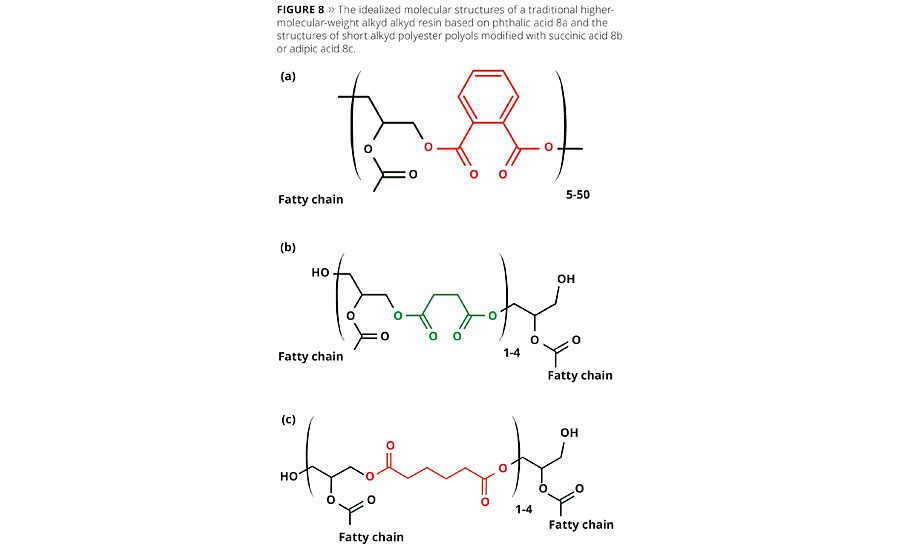
Figure 8 shows the generalized molecular structures of a traditional high-molecular-weight alkyd resin based on phthalic acid (8a) and idealized lower-molecular-weight building blocks based on succinic acid (8b) or adipic acid (8c). A series of building blocks based on structures 8b and 8c were prepared for this study. By adjusting the molecular weight and composition of these molecules, it was possible to design a biobased building block based on succinic acid, 8b, with a biobased content of around 75% to replace the AGS-based polyol. These polyols were then converted to stable, water-based dispersions containing around 40% solids using a reaction process similar to the one shown in Figure 7. The resins and subsequent dispersions were formulated into a final coating solution and were applied on Beech wood panels at 150 µm wet coating thickness and allowed to dry for 1 h at room temperature. The wood was then lightly sanded and a second coat was applied at 150 µm wet coating thickness and allowed to dry at room temperature for 7 days.
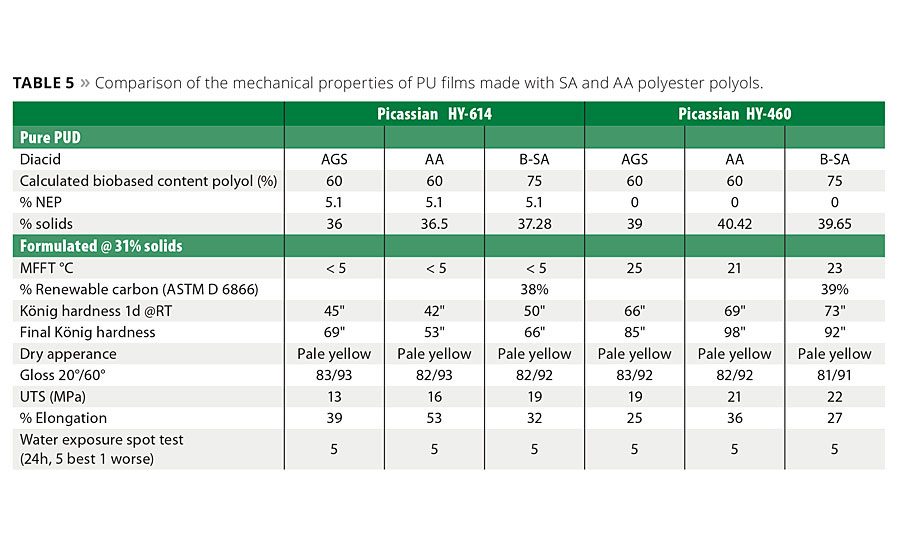
The coated samples were characterized using a variety of well-known and accepted coating performance tests including mechanical properties, hardness, water spot resistance, gloss and chemical resistance. The results of these side-by-side tests are summarized in Table 5. The desired outcome was to develop a replacement formulation that met or exceeded critical to quality (CTQ) metrics for optics, durability, application quality and final appearance of the coating. The biobased SA formulations for both HY-614 as HY-460 did produce a slightly harder final coating and slightly lower elongation. However, for a wood coating application the decrease in elongation is considered well within acceptable limits, especially because the other performance CTQs were well within the needed ranges and the final products had a significant increase in the biobased carbon content.
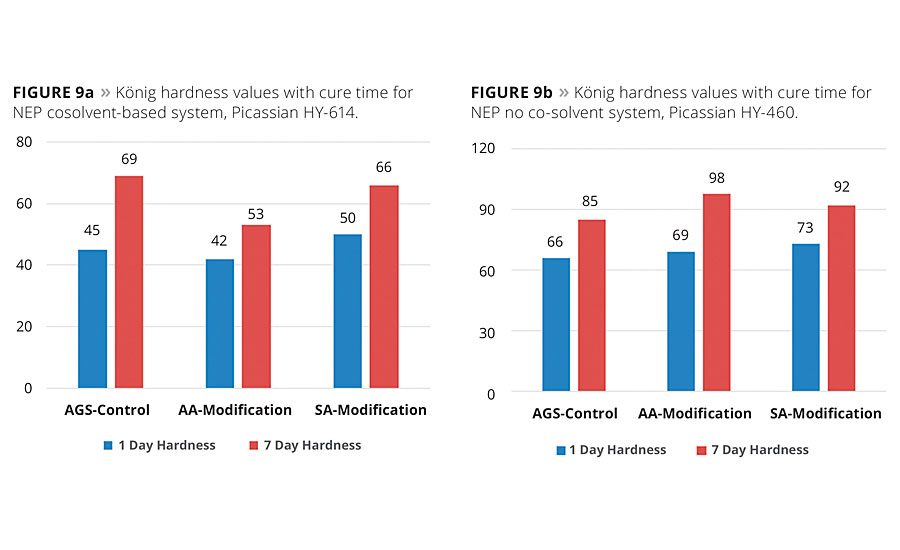
Figures 9a and 9b show the differences in surface hardness as measured on the König hardness scale between the two systems over the curing cycle. As is known in polyurethane chemistry, hydrogen bonds form slowly between the urethane groups and increase the hardness to a particular plateau hardness value based on the formulation. In the case of Picassian HY-614 (Figure 9a), the SA-based formulation exhibited nearly the same target hardness as the AGS-based control, and these were both more than 10 points higher than the petro-based AA formulation. In the case of the NEP cosolvent-free compositions (Figure 9b), both the SA and AA formulations exhibited slightly higher hardness values than the AGS control formulations.
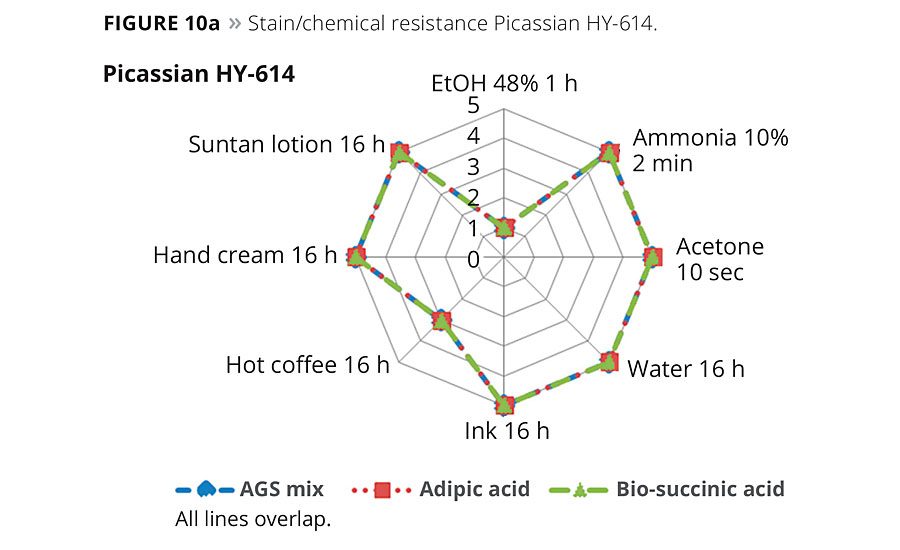
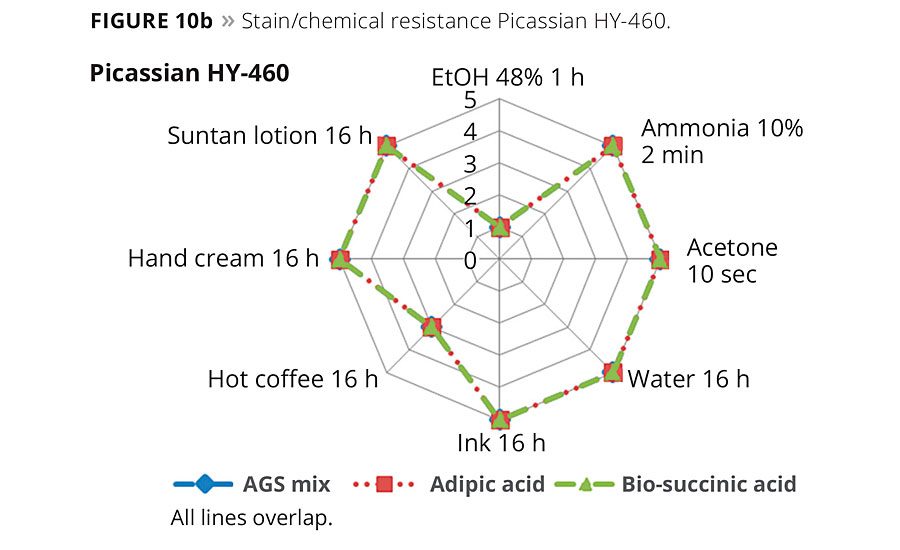
Figures 10a and 10b show radar graphs of the chemical and stain resistance of the final coatings after exposure to typical staining and cleaning media. The legends show the staining agent and exposure times. The numerical rating scale from 1-5 provides a value of worst (1) to best (5) performance of the coating to staining or damage by the reagent.11 As is clearly noted in Figures 10a and 10b, the two replacement formulations based on either biobased SA or petro-based AA are equivalent to the current product and show excellent performance in use. It should be noted that additional additives such as 3% of Stahl’s carbodiimide crosslinker Picassian XL-275 can improve the ethanol resistance to a rating of ≥4. However, the addition of crosslinkers needs additional mixing and is, therefore, less suitable for the DIY markets.
Conclusion
This article demonstrates the performance of biobased succinic acid in PUD and coating formulations. The generalized PUD coatings application study suggests polyester polyol succinates can improve solvent and abrasion resistance of urethane coatings and provide excellent performance and improved sustainability in urethanes. The development of a commercial uralkyd product utilizing biobased succinic acid suggests the generalized PUD findings are transferable to related technology areas, and renewable building blocks such as Bio-Amber’s biobased succinic acid can be formulated into cost-effective, high-performance coating products with excellent performance and value.
For more information, e-mail bill.coggio@bio-amber.com, visit www.bio-amber.com, e-mail frank.brouwer@stahl.com or visit www.stahl.com.
References
1 Coggio, W.D. et al. Bio-based Succinate Polyols in PUD Coatings (Part I). Presented at American Coating Conference April 7-9, 2014, Atlanta Georgia.
2 Coggio, W.D. et al. Structure-property Relationship of Polyester Succinate Polyols with Mixed Glycols. Presented at Urethane Technical Conference (UTECH-NA) June 3-5 2014, Charlotte, NC.
3 Coggio, W.D et al. Bio-Based Succinic Acid Polyester Polyols Sustainable Building Blocks for Performance Driven TPUs, PUDs, and Coatings. Presented at Council on Polyurethane Industry Conference (CPI Conference) Sept 22-24 2014, Dallas, TX.
4 Coggio, W.D. et al. Succinic Acid: A Bio-based Building Block in Performance Driven Polyurethane Dispersion for Coatings (PUD Study Part II). Presented at Waterborne Coating Conference Feb 11, 2105 New Orleans, LA.
5 BioAmber’s Sarnia Ontario Canada production facility is now under construction and will be mechanically complete by first quarter 2015 and fully commissioned by July 2015. BioAmber has announced “take or pay” agreements with Vinmar and PTT-MCC Biochem for both biobased succinic acid and bio-1,4-butanediol. See www.bio-amber/newsroom for more details.
6 Szycher, M. Handbook of Polyurethanes, 2nd Edition, CRC Press, Boca Raton, LA 2012.
7 Coggio, W.D.; Sonnait, M.O. Development of Low Color Alkyd Resins with High Content of Bio-Based Succinic Acid, PCI Magazine, Oct. 2014.
8 Wicks, D.A.; Wicks Jr., Z.W. Progress in Organic Coatings2005, 54, 141-149 and Hofland, A. Progress in Organic Coatings, 2012 73, 274-282.
9 Holmbert, K. High Solids Alkyd Resins, K. August 31, 1987 by CRC Press.
10 Approximate final coating formulations contained 86 wt% resin; 0.4% BYK 93-surfactant; 2% ethylene glycol, 2% butylene glycol– coalescing solvent; Taifgel PUR 61-viscosity modifier; water ~9%-adjust solids.
11 Chemical resistance rating scale, Rating = 1-significant coating damage, permanent damage visible. Rating = 5-no visible damage to the coating, staining agent removed without noticeable loss of the coating quality.
Acknowledgements
BioAmber and Bill Coggio would like to acknowledge the many helpful collaborations and efforts by Prof. Dean Webster and Ivan Hevus of the North Dakota State University, and Dr. Alan Schrock, Baylen Thomas and Ken Ulrich of the University of West Florida for their contributions to the BioAmber studies cited in References 1-4.
By William D. Coggio, Ph.D., Global Manager Applications and Technology Support, BioAmber Inc., Plymouth, MN | Frank Brouwer, Ph.D., Green Technology Chemist; and Xavier Roche, Research Chemist, Stahl International bv | and Edgar Alarcon, Applications Chemist, Stahl Polymers, Parets, Spain








Report Abusive Comment