In polymer dispersions the main phase is comprised of colloidal polymer particles that usually range in size between 1 nm and 1µm. These colloid particles are dispersed in a continuous phase (e.g., water).1 Industrial polymer dispersions normally contain 40-60% polymer. Typical properties associated with industrial polymer dispersions are usually free-flowing, with viscosity and shear rate based on the monomers used. Polymer dispersions are developed to meet the needs of the final application. Polymer films from dispersions are created following the evaporation of the water phase. The strength, elasticity and solvent stability depend on the chemical composition and the molecular weight of the polymer (Table 1). To increase the diversity of the polymer dispersion, copolymers of multiple monomers are manufactured to achieve additional properties.
Polymers (including all sorts of plastics) are widely used due to the different properties that can be obtained by the various types of polymerization processes and raw materials. In this article, the focus is on aqueous-based polymer dispersions.
Manufacturing Polymer Dispersions3, 9-11
Manufacturing polymer dispersions is a complex process based on free-radical polymerization. This emulsion polymerization process involves several stages and requires at least four key raw materials: water, monomer(s), emulsifiers and initiator(s). Additional raw materials are chain transfer agents, buffering agents, acids, bases and biocides. Chain transfer agents (e.g., mercaptans) are used to regulate the molar mass and the molar mass distribution of the polymer chains. Acids, bases and buffering agents are added to control the pH of the manufacturing process and of the emulsions as such. After production, biocides are added to the finished dispersion to control the microbiology.
After all components have been brought together, the polymerization process involves emulsification of the relatively hydrophobic monomer(s) in water by means of the added emulsifier, followed by the initiation reaction with either a water-soluble or an oil-soluble free-radical initiator. At the end of the polymerization, a milky fluid called “latex”, “synthetic latex” or “polymer dispersion” is obtained.
To start the whole free-radical polymerization process, radicals have to be generated. For this purpose, initiators are needed. Typical examples used in emulsion polymerization are persulfates, hydrogen peroxide, organic peroxides, azo compounds and persulfate-bisulfite. Initiators are activated by heat or are based on redox systems.
Once generated, the free-radicals react with the monomer(s) containing unsaturated carbon-carbon bonds to initiate the polymerization reaction. When the radical reacts with a monomer molecule a larger free-radical is formed which, in turn, reacts with another monomer molecule, thus propagating the polymer chain. Growing polymer chains are finally terminated (i.e., free electrons coupled) with another free radical, or with chain transfer agents, inhibitors, etc.
Emulsion polymerization can be done via a batch, semi-continuous or continuous manufacturing process. The most common manufacturing process is semi-continuous due to its flexibility in the control of heat generation, the properties and morphology of the polymer particles that enable the production of a more controlled chemical composition, and particle size distribution. It also makes it possible to achieve relatively high polymer quality such as homogeneous chemical composition and particle size distribution, which contribute to the rheology and film formation characteristic of the polymer.
Temperature is a critical factor when producing polymer dispersions. The process is carried out in a reactor that can reach temperatures as high as 90 °C in cases where a thermal initiator is used. When using a redox initiator, a temperature between 40-50 °C is usually needed. Further details on emulsion polymerization can be found in the Cherns3 and Yamak10 articles listed in the references.
Today, emulsion polymerization is an industrial process generating millions of tons of products. It produces high-molecular-weight colloidal polymers containing no or negligible VOCs. The reaction medium is usually water and this facilitates agitation and mass transfer, and provides an inherently safe process. With the conversion to water-based systems containing low to no VOC, the process is more environmentally friendly.
Impact of Microbial Contamination on the Polymer Dispersion12-13
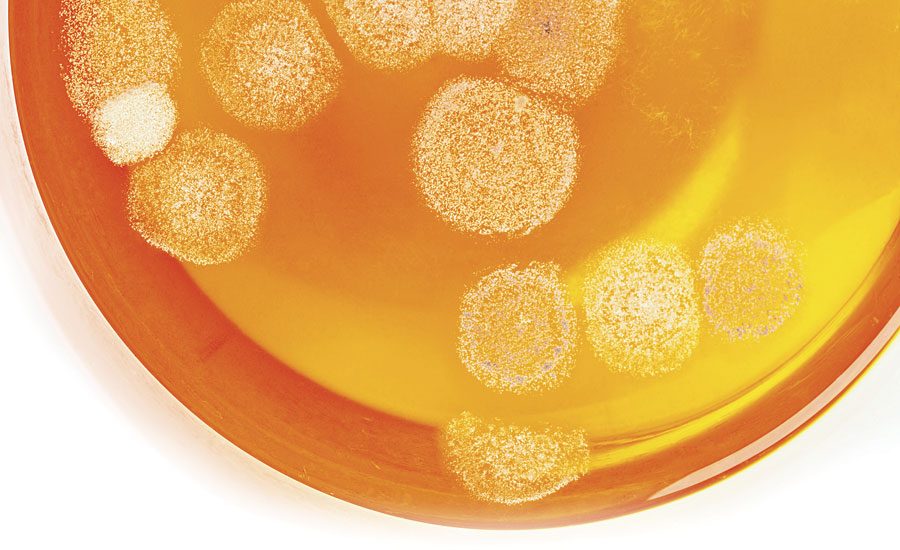
Polymer dispersions for the most part are aqueous-based formulations. The polymer dispersion is used in a wide variety of applications (e.g., adhesives and coatings). The composition of polymer dispersions with high water content and high nutrient level provides an environment that is perfect for the proliferation of microbial growth. Microbes and their metabolic byproducts can contribute to the breakdown of the polymer dispersion. Many factors are affected by the presence of microbes and their metabolic byproducts.
Water
Water is used as the medium in polymer dispersions because of its excellent heat transfer properties and its low viscosity. Water is also a fundamental growth factor for most microorganisms, with most needing a water activity above 0.50 for growth. Pure water has a water activity of 1.00. Pure water is not found in nature due to the solvent capabilities of the non-polar water molecule. In most industrial plants, water supply is the main source of microbial contamination.
Viscosity
Polymer dispersions can become thinner or thicker as a consequence of microbial growth (degradation of surfactants, acidic byproducts etc.) depending on the effect of the increased concentration of acidic byproducts. Phase separation can occur when a thinner top layer and a thicker bottom layer are formed. Since viscosity is a critical property of the finished product application, a contaminated polymer dispersion cannot be utilized in the finished product.
pH Change
As mentioned previously, the metabolic byproducts often are acidic in nature. The reduction in pH may cause a destabilization of the polymer dispersion. In addition, acidic conditions can promote a corrosive environment on the surface of the plant equipment.
Gas Production
As the microbes grow and multiply in the polymer dispersion, the fermentative bacterial cells will utilize the colloidal thickeners (e.g., cellulose, glucose) to produce organic acids and carbon dioxide. Gas generation is not usually noted in the plant environment but during storage of the polymer dispersion or finished product. Packaging distortion (expansion of the plastic bottle or pail) is usually observed in the warehouse.
Odor or Color Development
Polymer dispersions that are contaminated with sulfur-reducing bacteria can develop a “rotten egg” smell from the production of hydrogen sulfide gas. Sulfur-reducing bacteria can also blacken the polymer dispersion or finished product. Other microbes have the ability to produce odors based on their biochemical reactions.
Plant Equipment
The development of biofilms within the plant equipment (e.g., pipeline systems) can lead to increased production of microbial metabolic byproducts. In addition to all of the effects mentioned above, the diverse microbial communities within the biofilm can influence the corrosion of metal surfaces in the plant (i.e., microbial induced corrosion, MIC). It can also restrict the flow within the equipment piping, block filters and produce extracellular polymer that can generate foam.
Environmental Concerns
When there is a microbial contamination problem in the plant, the operators and plant personnel are exposed to foul odors and possible pathogenic microorganisms. Microbes are present in most plant environments. The type and the populations are affected by temperature, pH and type of nutrients. Aerobic bacteria, anaerobic bacteria (e.g., sulfate-reducing bacteria), mold and yeasts have been isolated from different polymer dispersion manufacturing facilities.
Factors Affecting Biocide Efficacy11-15
Redox Potential13, 15
Redox potential is a measure (in millivolts) of the affinity of a substance for electrons – its electronegativity – compared with a standard hydrogen electrode (SHE, which is set at 0).12 Radicals generated by oxidation-reduction reactions are commonly used to initiate the polymerization. The advantage to using this initiation process is the short induction period, low activation energy (10-20 kcal/mol) and the ability to control the polymerization reaction. Often used redox initiators are peroxides with reducing agents, inorganic reductants and inorganic oxidants (e.g., potassium peroxydisulfate, ammonium peroxysulfate), peroxydiphosphate systems (e.g., poly-merization of acrylo-nitrile) and organic-organic redox pairs ( e.g., alcohol by Ce4+).1 In the redox-initiated polymerization process, the reaction does not convert to 100% yield, leaving residual free monomer(s). In the finished polymer dispersion, residual free monomer(s) can react with the biocide active, reducing the biocidal efficacy. If the polymer dispersion has a positive redox potential, the product is in an oxidative situation. Biocides susceptible to oxidation may be degraded (e.g., benzisothiazolinone, BIT). In the presence of essential nutrients, aerobic bacteria can grow. In a negative redox potential environment, the polymer dispersion is in a reductive state. Biocides susceptible to reducing agents can be degraded. In the absence of oxygen and the availability of key nutrients, the polymer dispersion will provide an ideal environment for anaerobic bacteria. Table 2 illustrates the impact of redox potential on stability of the 1,2 benzisothiazolin-3-one (BIT) and the mixture of 5-chloro-2-methyl-4-isothiazolin-3-one and 2-methyl-4-isothiazolin-3-one (CMIT/MIT). A change in the stability of the biocide leads to a reduction in the performance.
pH
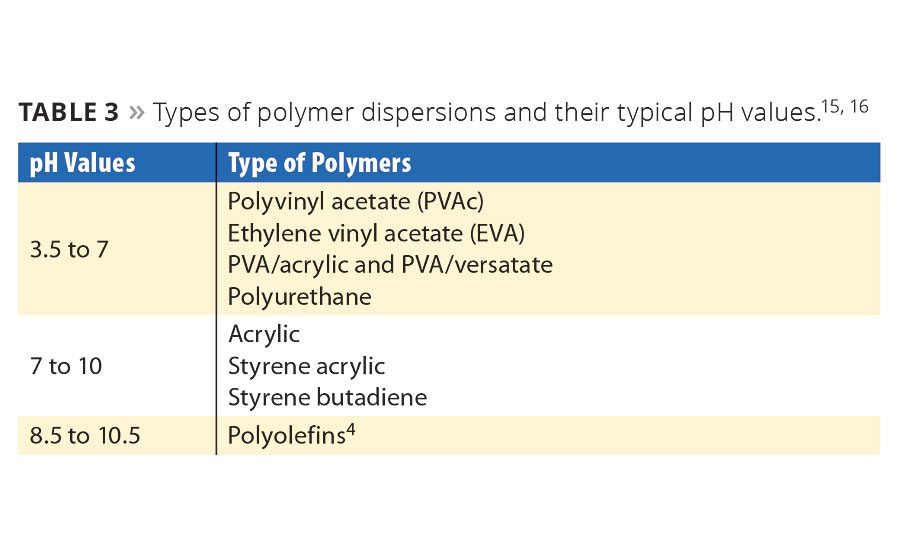
pH is critical to manufacturing polymer dispersions. In order to maintain a certain pH for optimizing the manufacturing of the specific polymer, a buffering agent may be added. Most of the microorganisms encountered in industrial plants grow within the 4-9 pH range. Fungal organisms are more prominent at acidic pH, and bacterial are more prominent at neutral to slightly alkaline pH. In general, polymer dispersions fall within the ideal pH range for microbial growth (Table 3).
Temperature
Temperatures in the polymerization process can reach as high as 90 °C. Most biocides, however, will degrade at higher temperatures and, therefore, are added after polymerization when the dispersion has been cooled down to a temperature acceptable for biocide addition. It is critical that the biocides be added at the earliest point after completion of the polymerization and after elimination of the free monomers. It is also important that the biocide is not added until the polymer dispersion temperature has cooled to a point that it will not break down the biocide. A biocide with a higher heat tolerance is recommended since polymer dispersion storage tanks are commonly insulated containers.
Other Components
Some components can further support the growth of microorganisms. Defoamers, metal catalysts and surfactants can be food sources for the microorganisms. Overall, the characteristics of an ideal biocide are broad-spectrum efficacy (effective against bacteria, mold and yeasts), effective over a wide pH range, temperature stable, resistant to redox agents, water soluble with partition coefficient that favors the water phase, compatible with other components of the finished product, good environmental profile, appropriate regulatory clearances and cost effectiveness.
Biocides Used in Polymer Dispersion Applications16-21
In the past, residual monomer(s) in the polymer dispersion provided some protection from microbe contamination. However, stricter environmental regulations and low to no residual monomer(s) in polymer dispersions provided a suitable environment for microbial growth. Biocides are needed to provide long-term protection against microbial contamination. Some of the preservatives currently used in polymer dispersions are BIT, BIT/MIT, CMIT/MIT, Bronopol , FA-R and MIT (Table 4).
BIT: 1,2-Benzisothiazolin-3-one (BIT) is an effective broad-spectrum microbiocide widely used in the polymer dispersion industry. It is stable at temperatures up to 100 °C. BIT provides biocidal efficacy at a wide range of pH (2-14). BIT does have a gap against Pseudomonas species, which are commonly found in industrial process waters. The mode of action for the BIT is that it reacts with the cell proteins causing the inhibition of respiration and ATP synthesis.17
CMIT/MIT: The three-to-one blend of methylchloro-isothiazolinone (CMIT) and methylisothiazolinone (MIT) is commonly used as a biocide in polymer dispersions. CMIT/MIT is a broad-spectrum biocide that works between 3-9 pH. The mode of action for the CMIT/MIT is that it reacts with the cell proteins causing the inhibition of respiration and ATP synthesis.20 Alkaline solutions degrade the CMIT molecule. CMIT has also been identified as a skin sensitizer at levels above 64 ppm.
BIT/MIT: The combination of 1,2-Benzisothiazolin-3-one (BIT) and methylisothiazolinone (MIT) provides a broad-spectrum bactericide for industrial products. The methylisothiazolinone closes the Pseudomona gap of the BIT. Since both BIT and MIT are also members of the isothiazolinone chemical family, the mode of action is that the chemicals react with the cell proteins causing the inhibition of respiration and ATP synthesis.20
Bronopol: 2-Bromo-2-nitro-propane-1,3 diol (Bronopol) is a bactericide that has some limited efficacy against fungal organisms. Bronopol is very effective against Pseudomonas species and should be used between a pH of 5 to 8.8 and below an application temperature of 45 °C. Bronopol has a complex mode of action that attacks the thiol groups within the cells inhibiting respiration and cell metabolism.21
FA-R: Formaldehyde-releasing biocides (FA-R) are industrial biocides used in paints, adhesives and concrete admixtures. They are mainly a bactericide but have some efficacy against fungal organisms at higher dosages. The mode of action for FA-R is that they release formaldehyde, which then reacts with nucleic acids and amino acids in the cell.21
MIT: Methylisothiazolinone (MIT) is an industrial bactericide used in paints, adhesives and cosmetics products. It is an effective bactericide with limited fungicidal efficacy. The mode of action for the MIT is that it reacts with the cell proteins causing the inhibition of respiration and ATP synthesis.20
Recently, dodecylguanidine hydrochloride (DGH) has shown biocidal efficacy against microorganisms in specific polymer emulsions (e.g., polyvinyl acetate-co-ethylene), which are free of anionic surfactants. DGH is distinguished by a remarkable activity against Gluconoacetobacter liquefans, as described in US6890969.22
Selection of the biocide is critical to the stability of the polymer dispersion. Contamination from the environment, raw materials, water, poor plant hygiene and dirty storage containers can lead to microbiological problems. Polymers that are not adequately preserved can lose or build their viscosity, form gases, develop foul odors, become more acidic, break the polymer dispersion, change colors and lose their polymer properties.
Global Regulatory/ Environmental Concerns
As consumers have become more aware of chemistries used in their paints, adhesives and sealants, stricter regulations have been placed on commercial products (e.g., low VOCs and non-skin sensitizers). Selection of an appropriate biocide for a given polymer dispersion due to the complex finished applications can be challenging. Polymer dispersions going into the food sector especially, must comply with strict food/law regulations.
Biocides used in the United States are regulated by the EPA. All biocides used to preserve industrial products must be registered under the Federal Insecticide, Fungicide and Rodenticide Act. Extensive data is required to support federal and state registration of the biocide (e.g., toxicity, ecotoxicity, physical and chemical properties and efficacy testing for each application area).
In Europe, the selection of biocides used in aqueous polymer dispersions is limited to the active substances approved by the EU, needs to be effective and cannot affect the properties of the finished application product in which the polymer dispersion is used.
In September 2014, the proposed Chinese biocide regulations were outlined at a Summit in Singapore. These include: remove the temporary registration phase, a licensing system for the trade of pesticides and biocides, a system for waste management of biocidal and pesticidal products along with the development of a recycling system, establish a recall system and the recall enforcement program, re-evaluation of products over 20 years old and collection of penalties for the violation of these regulations.23
Conclusions/Recommendations
The incorporation of the biocide shortly after polymerization can mitigate the risk of degradation of the biocidal active. The addition of the biocide too early could lead to polymer dispersion with insufficient levels of biocide during the loading, transportation, storage and finished product life cycle.
Addition of the biocide should occur when the temperature is as low as possible (<50 °C) and the lowest possible level of free radicals is in the polymer dispersion. Active levels of the biocides in the polymer dispersion should be monitored by high-performance liquid chromatography. Measuring the redox potential is also suggested. A concentration versus time curve can help provide essential information.
Due to the susceptibility of the polymer dispersion to microbial contamination, it is also recommended that a plant hygiene audit be performed. The Plant Hygiene Audit can identify areas of possible contamination points and will complement well with the performance of the biocide program chosen for the polymer dispersion.
References
1 Tauer, K. Polymer Dispersions. Series: Progress in Colloid and Polymer Science, Vol. 124. (Ed.) 2004, VII, p.170.
2 Urban, D.; Takamura, K. Polymer Dispersions and Their Industrial Application, 2002 Wiley VCH, p.6.
3 Chern, C.S. Emulsion Polymerization Mechanisms and Kinectics, Progress in Polymer Science, Vol 31(2006) p. 443?486.
4 Diehl, C.F. et al. Waterborne Mechnical Dispersions of Polyolefins, The Dow Chemical Company, Form #789-00801-0507 obtained on www.dow.com/webapps /lit/litorder.asp.
5 http://www.bpf.co.uk/Plastipedia/Polymers/Polyesters.aspx.
6 http://www.pvc.org/en/p/pvcs-physical-properties.
7 http://www.buzzle.com/articles/polystyrene-properties.html.
8 nzic.org.nz/ChemProcesses/polymers/10C.pdf, Compiled by Roger Glanville (Unitec Auckland) following an interview with Bobby Kanji (Rohm and Haas New Zealand). Edited by Heather Wansbrough following further communication with Bobby Kanji.
9 Anderson C.D.; Daniels, E.S. Emulsion Polymerisation and Latex Applications, ort 160, ISSN: 0889-3144 Volume Number 4, 2003.
10 Yamak, H.B. Emulsion Polymerization: Effects of Polymerization Variables on The Properties of Vinyl Acetate Based Emulsion Polymers. Polymer Science, p. 35-72 January 23, 2013.
11 Gillatt, J.W. The Microbial Spoilage of Polymer Dispersions and its Prevention in Directory of Microbicides for the Protection of Materials, A Handbook Edited by Wilfried Paulus p.219-249.
12 Cresswell, M.A.; Holland K. Preservation of Aqueous-Based Synthetic Polymer Emulsions and Adhesive Formulations, Preservation of Surfactant Formulations, Chapman & Hall 1995 p. 232-236.
13 Gillatt, J. The Biodeterioration of Polymer Emulsions and its prevention with Biocides, International Biodeterioration 1990, Volume 26, Issues 2-4, Pages 205-216.
14 Redox Potential Home Page. http://users.rcn.com/jkimball.ma.ultranet/Biology Pages/R/RedoxPotentials.html (Access October 24, 2014).
15 Gillatt, J. The Effect of Redox Chemistry on the Efficacy of Biocides in Polymer Emulsions.
16 LANXESS Product Information Sheet, Biochek® BIT 20, 2007-04-17.
17 Microbicides for the Protection of Materials, A Handbook Edited by Wilfried Paulus Part Two, p.659-661.
18 LANXESS Product Information Sheet, Preventol® P100 Preservative, 2008-03-31.
19 Form No. 253-03194-09/19/13 PS Dow Microbial Product Brochure Neolone™ 950 Preservative.
20 Williams, T.M. The Mechanism of Action of Isothiazolone Biocides, PowerPlant Chemistry 2007, 9(1) p.14-22.
21 Myers, F. Biocidal Agents: Modes of Action and Correlation with Antibiotic Resistance, The Biomedical Scientist, March 2008 p.227-231.
22 Rabasco, J.J.; Sagl, D. Polymer Emulsion Preservation using Cationic Compounds. U.S. Pat. No.6,890,969, May 10, 2005.
23 Industry expects stricter regulation of biocides in China, Chemical Watch, September 2, 2014.
This paper was presented at the 2015 Waterborne Symposium. For more information, contact LANXESS Corporation, 111 RIDC Park West Dr., Pittsburgh, PA 15275; Phone: 1-800-LANXESS, www.lanxess.com.






Report Abusive Comment