Acrylic Hybrid technology
To design higher performance surface coatings, coating formulators must choose from myriad polymer chemistries. The polymer holds the coating cohesively (internally) together and adhesively (externally) to the substrate to which it is applied. Proper polymer selection can be the most important factor in determining the physical and chemical properties, as well as protective capabilities, of the coating.
In addition to desirable qualities, each polymer typically has shortcomings. Polymer manufacturers have developed hybrid polymer technology to combine the advantages of one polymer species with another - while offsetting their shortcomings - in a synergistic manner to create a higher performance class of polymers. As a novel class of chemistry, hybrid polymer technology gives coating formulators new tools to compound continuously higher performance systems.
This article discusses acrylic hybrid technology, the broad application of this class of polymer species and, in particular, the design and development of non-yellowing, high performance coatings using acrylic hybrid polymers.
Applications
Acrylics deliver UV resistance, gloss retention and improved adhesion in a range of coatings. They are versatile polymers, available in a variety of forms: thermoplastic, thermosetting, aqueous- or solvent-soluble, solution, emulsion, powder, and radiation-curable. They offer chemical resistance, are compatible with many resins and can be formulated with low VOCs. Monomer variations enable development of customized copolymers to meet specific application requirements. Costs are moderate.These qualities have contributed to widespread success for acrylics. Based on acrylic and/or methacrylic polymers or copolymers, global annual sales of acrylic coatings has reached the $10 billion range. Acrylic surface coatings are vehicles in three coatings market categories: architectural, OEM and special-purpose coatings. Acrylic polymers are also preferred for protective fabric and leather finishes, floor polishes, and paper coatings. Future growth will result primarily from increasing demand for high-solids and waterborne formulations driven by regulatory mandates for lower VOC emissions.
What is an Acrylic Hybrid?
An acrylic hybrid coating is prepared by reacting an acrylic polymer with a large portion of another functional polymer to produce a coating that combines the beneficial properties of both the acrylic and the other functional polymer. Often, physical blends or chemical reaction of two different polymer systems will provide an incompatible mixture that exhibits the worst properties of each polymer. The polymer manufacturer's expertise resides in the ability to design properly formulated acrylic hybrids that yield compatible mixtures of the acrylics with the other functional polymers. Many examples of acrylic hybrids have been noted.1-7Advances in Acrylic Hybrid Technologies
It is crucial in any acrylic hybrid to ensure compatibility among all polymers in the hybrid through physical blending or chemical reaction between the components of the hybrid. Once this has been accomplished, acrylic hybrids can be developed to bring the best qualities of each polymer to a new coating. For example:- Acrylic - Hardness, gloss, weatherability, fast dry or tack-free time, crosslink ability (from reactive monomer functionality - OH, acid or epoxy groups, etc.).
Epoxy - Adhesion, toughness, corrosion protection, solvent resistance.
Urethane - Flexibility, abrasion resistance, acid rain resistance, gloss retention, impact resistance.8
Silicones - Durability, heat resistance, flexibility, anti-graffiti/stain resistance, release coating.5,9
Guidelines for Acrylic Hybrid Selection
The appearance of more acrylic hybrids demonstrates the versatility of combining acrylics with other functional polymers. It is essential in any acrylic hybrid to develop compatibility between the acrylic and its functional hybrid partner. Following are a few examples of acrylic hybrids.- 1) Alkyd Acrylic Hybrid - The most common acrylic hybrids for alkyd modification are physical blends of hard acrylic copolymers with a softer short or medium oil alkyd for general industrial spray coating. The acrylic provides quick drying, low tack, toughness and durability. The alkyd provides self-priming and air drying to enhance solvent and chemical resistance, compared to a thermoplastic lacquer.
2) Epoxy Acrylic Hybrid - The high gloss, hardness, fast dry and exterior durability of an acrylic with the desirable chemical resistance and adhesion of epoxies has led to acrylic/epoxy hybrids. Early acid-functional acrylics, crosslinked with either glycidyl bisphenol-A or epoxidized soybean oil, produced interesting baking enamels. Recent emphasis has focused on catalyzed room temperature curing acid acrylics with epoxy resins.
3) Silicone Acrylic Hybrids - Siloxane acrylics were developed as durable coil coating finishes. The siloxane was post-reacted with a preformed hydroxyl acrylate and crosslinked with added aminoplast during application. Today, hybrid systems are available for clearcoat automotive application based on reactive alkoxy-silanes copolymerized with acrylic monomers and crosslinked during application with a polyisocyanate to give superior acid etch, water spot and mar resistance.5
4) Dual Curing Acrylics - Dual-cure types, such as isocyanate-modified acrylics with latent unsaturation for combination UV and hydroxyl/isocyanate curing, are appearing as extensions of simple hybrids to solve coating problems. For example, the absence of UV curing in shaded areas can be overcome by adding a second crosslinking mechanism.10
Guidelines for Acrylic Hybrid Preparation
To synthesize an acrylic hybrid, a base acrylic with functionality must be prepared to couple or "compatibilize" with its hybrid functional polymer. The base acrylic of the hybrid is designed with the optimal acrylic film properties required for the final cured coating as well as the appropriate functional properties (either to assist the hybridization with the other functional polymer or to provide curing sites for crosslinking during final film curing). Courtesy of Dock Resins Corp.
Courtesy of Dock Resins Corp.
Base Acrylic Design
The design of the base acrylic is the primary determinant of coating performance. Monomers are selected according to the desired final film properties. For example, methyl methacrylate is chosen for gloss and hardness. Often styrene is incorporated for additional hardness, gloss and compatibility with some solvents. For acrylic backbone strength, flexibility and toughness, butyl methacrylate or 2-ethylhexyl methacrylate are often considered. If flexibility is desired, butyl acrylate or ethyl acrylate are possible co-monomer choices. Typically a balance of hard and soft monomers is selected dependent on final film hardness, toughness, and durability.Monomer selection is important for final film performance in exterior application. For example, vinyl toluene and styrene providing initial high gloss may lead to yellowing and poor gloss retention if added in large amounts (greater than 20%). Branched acrylates - 2-ethylhexyl acrylate will lead to extra gloss loss on exterior exposure vs. butyl acrylate. Overall, acrylates and methacrylates provide excellent gloss retention and durability, particularly straight chain methacrylates - butyl or ethyl methacrylate. Table 1 indicates the effects of monomer selection properties on film/coating properties.
Functional comonomers for hybridization through physical interaction with another polymer include acrylic acid, acrylamide or dimethyl aminomethyl methacrylate. For chemical interaction with the functional polymer, many co-monomers are selected, depending on the coupling mechanism required as indicated by examples in Table 2.
 Courtesy of Dock Resins Corp.
Courtesy of Dock Resins Corp.Catalysts, Solvents and Molecular Weight
The molecular weight of the final acrylic polymer is crucial for effective hybridization. Lower molecular weights are favored, as is low polydispersity (Mw/Mn - close or equal to 1). These polymers require careful selection of initiators. For example, azonitrile initiators, (2,2'-azobisisobutyronitrile) and peroxyesters, (t-amyl peroxy-2-ethylhexanoate) provide initiation at desired temperatures for reaction control.Half-life of initiators is crucial for requisite monomer conversion. Typical criteria include providing at least three half-lives during the time of polymerization. Mixtures of initiator types, including post-addition of higher half-life materials, are often required to reduce residual monomer levels. Initiator selection is particularly important where high exterior durability is required. Here, the formulator must select initiators to ensure minimum decomposition byproducts that can affect durability. Also, volatile byproducts can lead to blistering, voiding or cratering in final cured films.
Many variables in acrylic hybrid preparation contribute to its versatility. Besides monomer selection, the choice of initiator, solvents, polymerization temperature and pressure affect the molecular weight. Impurities from initiator decomposition and residual solvents can impact final film performance - including shrinkage, yellowing and surface defects. The kinds of curing mechanisms for hybrid formation also impact final film performance. For example, an amide/acid hybrid may give poor weathering characteristics but may be excellent for metal primer application.
 Courtesy of Dock Resins Corp.
Courtesy of Dock Resins Corp.Hybrid Formation
Hybrids can be formed during or after the base acrylic polymer preparation. Sometimes it is advantageous either through free-radical copolymerization, chemical condensation or chemical addition to form the hybrid during co-polymerization of the base acrylic. Hybrid acrylic urethanes and hybrid acrylic siloxanes can be formed in this manner. On the other hand, some urethane acrylates, polyester acrylates, and siloxane acrylates are formed by chemical or addition reaction after the base acrylic formation. Each of these techniques can provide unique properties to maximize compatibility and final film curing properties of the acrylic and the hybrid functions.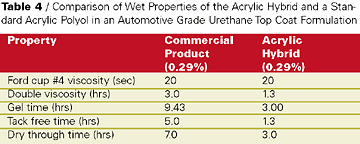 Courtesy of Dock Resins Corp.
Courtesy of Dock Resins Corp.Experiments in Designing Acrylic Hybrid Resins
A comparative study illustrates the importance of monomer, catalyst and solvent selection in developing the desired properties of simple thermoplastic acrylics. We learned by this study the importance of the base acrylic design in any copolymer system. This design is also important, which we will show by analyzing the film properties of a proprietary acrylic hybrid that is subsequently crosslinked with an isocyanate biuret prepolymer.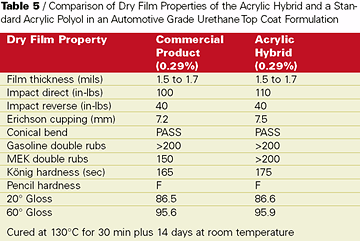 Courtesy of Dock Resins Corp.
Courtesy of Dock Resins Corp.Base Acrylic Design: Styrene and Catalyst Effects
With some simple base styrene acrylates and an acrylic control, we conducted an experiment to determine factors influencing the degree of color change and yellowness in a series of copolymers for concrete cure and seal products. A well-respected independent ISO-certified laboratory was selected to perform a QUV weatherability study (see Table 3). Five liquid samples labeled A-E, adjusted to similar percent solids, were submitted in a blind study for artificial weathering in accordance with ASTM G 53 for 300 hours. The exposure cycle was 8 hours UV at 70 deg C, using UVB 313 bulbs, followed by 4 hours of condensation at 50 deg C.Most solventborne cure and seal concrete coatings are based on thermoplastic styrene acrylate copolymers for strengthening freshly poured concrete or sealing older concrete surfaces. However, one unwanted side effect of styrenated acrylate copolymers is their tendency to yellow from sunlight exposure. Though all-acrylic methacrylate polymers are promoted as non-yellowing alternatives, they do not meet the ASTM C 1315 and C 309 standards for curing and sealing concrete. They typically add gloss, chemical resistance and overall surface protection to topcoating sealers.
 Courtesy of Dock Resins Corp.
Courtesy of Dock Resins Corp.Results
Since all styrene acrylate copolymer systems tend to yellow from direct sunlight exposure, one goal of the concrete curing industry is to lower yellowing side effects of cure and seal products. As styrene content rises, yellowing increases. Less obvious factors also influence yellowing. Free radical initiators from solution polymerization of styrene acrylic copolymers may influence the degree of yellowing. Minimizing impurities of aromatic byproducts from initiator decomposition may lower the tendency to yellow from direct sunlight exposure. The results of the limited QUV exposure study suggest a direct correlation between styrene content, types of initiators used, their concentration and the resulting aromatic catalyst byproduct levels remaining in the styrenated polymer film.Table 3 demonstrates that styrenated acrylate copolymers have a significantly greater increase in yellowness upon artificial weathering compared to all-acrylated copolymers (Sample B is the only all-acrylic sample). The primary cause of discoloration upon QUV exposure is the styrene monomer and the varying amounts of byproducts.
Non-Yellowing High-Performance Acrylic Hybrid
The knowledge obtained about base acrylic design - particularly initiator, molecular weight, and solvent - in the concrete cure and seal application was applied successfully to develop a topcoat for an automotive industry customer. They wanted a harder room-temperature air-dry automotive refinish system but with non-yellowing properties equal to a pure acrylic. Building hardness would potentially sacrifice some of the non-yellowing of a pure acrylic polymer. An acrylic hybrid was developed that incorporated during copolymerization a proprietary functional hybrid to improve the hardness of final cured films while preserving the non-yellowing properties desired in the auto refinishing coating.This acrylic hybrid resin was formulated in a two-component hydroxyl acrylate/isocyanate curing formulation (see Table 4). The curing agent in this study was an isophorone diisocyante based polyisocyanate curing agent. The Tg of the acrylic hybrid resin was measured to be about 10 deg C higher than a pure hydroxyl acrylate. Coating formulations of the acrylic hybrid were formulated to 55%-solids in an exempt solvent level blend and compared to a well-known commercial product in auto refinishing application. Dry film properties of catalyzed elevated temperature cured films showed equal film performance with slightly harder films from the acrylic hybrid (see Table 5).
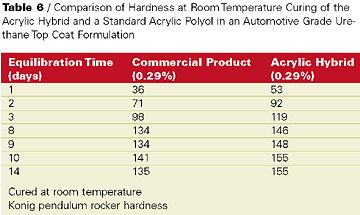
Also, the acrylic hybrid demonstrated faster tack free time and dry through than the commercial product. Most impressive were the KEnig pendulum rocker hardness values over time of the proprietary acrylic hybrid film compared to the commercial product in films cured at room temperature (see Table 6).
These studies demonstrate the power of acrylic hybrids to improve performance of base acrylic polymer systems. We have examined in this study only one proprietary acrylic hybrid system.
Polymer companies can create finely tuned new base acrylics and then a variety of acrylic hybrids with performance tailored to the needs of the marketplace.
Future Trends for Acrylic Hybrid Polymers
What does the future hold for multifunctional acrylic hybrids? The literature is revealing exciting trends for broad application of acrylic hybrid technology.- a) A hard, protective, abrasion-resistant coating for a thermoplastic polycarbonate surface, developed from a polyalkoxysilane acrylic monomer combined and reacted with colloidal silica and cured with a multi-functional monomer;10
b) Nanoparticles derived from aqueous sodium silicate sol-gel technology have enabled the delivery of silica nanoparticles in various polymers and oligomers without affecting viscosities. Once cured, the nanoparticle-containing hybrid polymers have improved scratch and abrasion resistance;11 and
c) Recent literature has appeared on germicidal functional coatings.12
The acrylic hybrid base with vast formulating latitude enables exploration of a diverse range of hybrid chemistries. Future acrylic hybrids will combine pigment and filler into hybrid structures, join with nanochemistry to provide enhanced film properties, and include inorganic acrylic hybrid structures - all providing new and unique coating benefits.
For more information on acrylic hybrids, contact Dock Resins Corp., 1512 West Elizabeth Ave., Linden, NJ 07036; phone 908/862.2351; fax 908/862.4015; visit www.dockresins.com; or e-mail sales@dockresins.com.
References
1 Wicks, Z.; Jones, F.N.; Pappas, S.P. J. Coatings Technol., 71, # 892, 41 (1999).deJongh, R.; Visser, A.; Van der Linde, R . Eur. Patent Applic. 10805 (1980), Chem Abstr. 94, 141345u (1981).2 Graver, R.B. J. Paint Technol., 39, #505 71 (1967).
3 Potter, T.A.; Williams, J.L. J. Coatings Technol., 59, #749, 63 (1987).
4 Eslinger, D.R. Modern Paint & Coatings, April, 2000, p. 21.
5 Lin, J.; David Nordstrom, J. US 5985463 (1999).
6 Kondos, C.; McEntire, E.E.; Nakajima, M.; Nugent Jr., R.M.US 6203913 (2001).
7 Skinner, E.; Emeott, M.; Jevne, A. US 4247578 (1981).
8 Courter, J.L. J. Coatings Technol., 69, #866, 57 (1997).
9 Feldmann-Krane, G.; Josten, W.; Lanngenhagen, R.; Reusmann, G.; Silber, S.; Spratte, W.; Stadtmuller, S. US 6297331 (2001).
10 Medford, G.F.; Gillette, G.R.; Sato, N. US 5827923 (1998). Medford, G.R.; Patel, G. US 5708048 (1998)
11 Adebahr, T.; Roscher, C.; Adam, J. European Coatings J., April, 2001, p. 144.
12 C&EN, June 10, 2002, p. 36.
13 Protective Coatings, Fundamentals of Chemistry and Composition, Clive H. Hare, 1998, p. 97.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!








