
The Fourth Dimension of Silicon: Siltech Q Resins
Silicone, or PDMS, is a linear, two-dimensional polymer. This well-known, widely used substance is a very low Tg liquid that is insoluble in both oil and water. Silicone materials typically impart wetting, shine, softness, and release when used in a formulation. They also impart flexibility, softness, haptic properties, and low surface energy to the network when reacted with another polymer.
In silicone chemists’ jargon, the end units of these silicones are M groups and the middle, repeat units are D groups. Other Si-O bond species are T and Q moieties. A pneumonic device for remembering the labels is that they refer to the number of Oxygen atoms attached to the Silicon atom. So, M and D refer to mono and “di” Oxygen bonds while the T and Q have three and four Oxygen bonds respectively.
In the CASE industry, we are also very familiar with T groups. Most often found in the guise of trialkoxy organofunctional silanes, these are used to improve adhesion. This is especially common when bonding organic and inorganic substances. The silane works by reacting some of the three Si-O bonds with the mineral-like species and the organofunctional groups with the organic substance. These are also commonly used to stabilize mineral dispersions, and to modify polymers providing enhanced properties from increased cross-link density. Other applications exist for trialkoxysilanes, but they all rely on the reactivity of the T group.
At Siltech we have a line of Silmer TMS polymers that are silicones modified with a trialkoxy silane either at the ends of the polymer or in the repeat units. These materials provide unique characteristics including secondary cure, stain repellence, and in some cases oil repellence. When combined with silicas super hydrophobic properties were observed. To read a good overview of these “evolved silanes” see Dr. Mike Gunther’s overviews on our website.
Less well known are species that have a high degree of Q character. This Q group is a structural unit in which the Si atom is bonded with all four bonds to Oxygen. Unlike the more easily accessible T networks, networks formed with a lot of Q character tend to be reasonably stable to hydrolysis and other reactions. It is one way to obtain much of the performance of a silicone without the ease of hydrolysis, which sometimes causes problems in a formulation.
These Q-based species have been used for years, but often travel incognito and in small percentages. MQ resins, in which the Q network is terminated with trimethylsiloxy groups have been used extensively for feel, transfer resistance, and cushion in cosmetics as well as anti-squeak and lubrication in industrial applications. Another very big application has been in antifoam compounds that require a high level of durability such as pulp and paper, laundry, and chemical processing applications. In these applications, the chemical stability of the MQ resin is a critical property to its success.
We at Siltech have spent years developing reproducible processes to make various Q resins that contain M, D and/or T groups. Unlike silicones, these materials are not allowed to react to a thermodynamic energy minimum. Instead, they are kinetic products and the rigor and precision of the process are critical to the reproducibility and consistency of the final product. In other words, these are tricky to make and difficult to copy.
After developing the processes, we searched for unique properties and applications of these materials. We have found that when used in a mixture, these Q resin species provide exceptional water repellence, stain repellence, release properties, and in some cases, oil repellency. They also provide tear resistance and strength when reacted into another resin system. This is often a complement to the properties brought by reacting two-dimensional silicones into a resin network.
As an example, using MDTQ resins in silicone networks such as elastomers, or sealants can restore some of the inherent deficiencies of these systems. Specifically, Silmer VQ and Silmer H resins systems are available with vinyl groups and Si-H bonds respectively. When used in addition cured elastomers these tend to bring strength and tear resistance. While silica can also do this, the Q resin approach works at lower use levels and retains the transparency of the final product.
Most recently and in the context of developing PFAS alternatives, we have been experimenting with the idea that these T and especially the Q containing resins can self-assemble and react in place providing super-hydrophobic properties. A succession of observations in which monofunctional Q resins or difunctional silicones gave oleophobicity led us to the theory that these species are assembling.
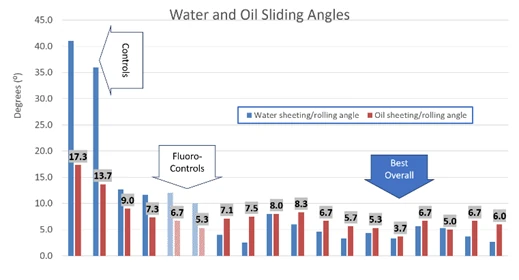
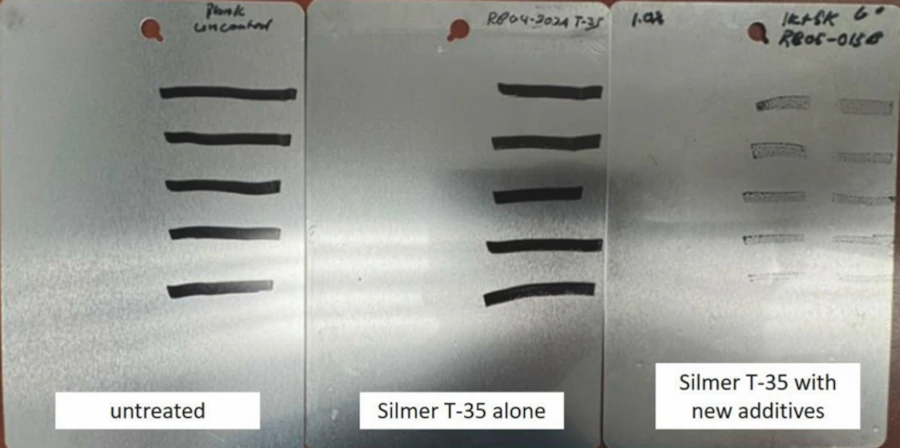
Very recent work has included a difficult to manufacture type of monofunctional silicone locked into Siltech T35, a Q resin matrix. This system has shown significant improvements in the receding contact angles of mineral oil as well as very good marker release over a fluorosilicone control.
Future work will continue to improve on oleophobicity by fine-tuning this assembly approach. If you are interested in more detail, please contact us or come to see John Grande, one of our chemists, who is presenting this work at the Coatings Trends and Technologies Summit and the Western Coatings Symposium.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!







