The Effect Of Rinse Water Quality On Powder Coating Corrosion Resistance
 Courtesy of Morton Powder Coatings and Rohm & Haas
Courtesy of Morton Powder Coatings and Rohm & HaasConsumer expectations define actual coating performance requirements. Finishing systems should be closely monitored and controlled, with systematic part performance testing to ensure consistent quality to the end-user. Coatings and pretreatment suppliers can assist this process by providing salt spray and other forms of physical property testing.
Chemical Systems
Chemical suppliers offer a variety of aqueous cleaners and conversion coatings designed to prepare metal parts to receive powder coatings. These metal treatments are most typically applied in a multi-stage spray washer. Pretreatment chemicals remove organic and inorganic soils (oil, grease, polymers, soaps, oxides and particulate) from metal parts that would prevent powder coatings from mechanically bonding to the substrate. Iron or zinc phosphate conversion coatings promote powder adhesion by creating a surface structure. The phosphate conversion coating also enhances corrosion resistance and limits flash rust.Every year, metal finishers invest heavily in chemical treatments and monitoring temperature, titration and pH of the pretreatment process. However, it is not uncommon for metal finishers to view pretreatment as a “black box” process — chemicals and dirty parts in and clean conversion coated parts out.
Other factors significantly impact coating and pretreatment performance. First, general washer mechanical condition including pressure, nozzle condition and nozzle placement should also be monitored because good impingement is essential for good cleaning. Second, operating parameters including time and temperature must be defined and closely followed, and third, quality conscious metal finishers must carefully monitor rinse water, total dissolved solids (TDS) and pH.
Good water quality is fundamental to any aqueous pretreatment system. Excessive water hardness and TDS limit the effectiveness of chemical stages, leading to greater chemical usage.1 Softened water is not the answer; the softening process simply replaces sodium for heavier elements in the water. Any sodium remaining on the metal surface can serve as an initiator for the corrosion process, and a potential cause of coating delamination.
Metal finishers must recognize that dissolved solids in the water will be deposited on metal parts during rinsing. The same effect occurs even if the final rinse contains an acidified sealer. When the water evaporates, chlorides, sulfates and calcium salts, along with other chemicals and minerals present in the water, will remain on the metal substrate. In many instances, the final stage of a spray washer is a water rinse. De-ionized water produces the best results, but unfiltered municipal water can produce adequate results if dissolved solids are controlled within acceptable limits.
Organic Coatings
Organic coatings act as a semi-permeable membrane, allowing moisture to migrate through the polymer film as a result of osmotic pressure.2 When mechanical adhesion of a powder coating to the substrate is impaired, moisture readily permeates the coating film and causes it to swell, thus creating a corrosion site and lifting the coating film away from the substrate. Coatings with high levels of inert extenders or low crosslink density will be more permeable and thus more susceptible to corrosion. As a side note, coatings heated above the glass-transition temperature (Tg) will be more permeable as the increased molecular motion will allow moisture to pass through the film more easily.As the coating respires, hygroscopic salts left on the substrate will absorb transmitted moisture until the salts resoluablize, breaking the weak mechanical bonds that may have existed with the deposited matter and the coating substrate interface. Regardless, the end result is the same: coating delamination and substrate degradation. This effect can be particularly significant on drip lines and other spots where rinse water puddles prior to drying, causing a concentration of solids to form. As a note, the latency of adhesion problems can be most frustrating for metal finishers. Coating performance related to poor rinsing may deteriorate over days or weeks after coating application. As a result, the coated parts can pass adhesion testing one day and fail the next.
Rinse Water Purity
Powder coating adhesion can be significantly impeded when dissolved solids are transmitted to the parts in rinse water. Published recommendations3 for TDS in system source water are <300 ppm. Another author4 recommends that water with greater than 250 ppm of solids as CaCO3 (moderately hard) not be used as source water for a final sealer rinse. Additionally, the total of combined chlorides and sulfates should not exceed 100 ppm.Although these recommended levels of water purity yield satisfactory performance for many applications, the research that follows will show that superior performance is possible with lower TDS. It should not be assumed that municipal water districts provide consistent water purity over time. TDS levels in source water vary significantly as different water sources (well, river, desalination) are used to meet spikes in demand. TDS levels should be diligently monitored and recorded.
 Courtesy of Morton Powder Coatings and Rohm & Haas
Courtesy of Morton Powder Coatings and Rohm & HaasExperiment
To facilitate the experiment, 20 steel test panels were prepared by ACT Laboratories Inc.5 per the Bonderite 1000®,6 P-60 process outlined below.- alkaline clean
- hot rinse
- hot rinse
- cool rinse
- iron phosphate (B-1000)
- ambient rinse
- chrome sealer
- deionized water rinse
- dry off
A comprehensive chemical analysis of a tap water completed by the Water Quality Association, Naperville, IL,7 identified common elements in tap water. The following listing summarizes the six most common metals and ions present in the tap water sample in descending order.
- CaCO3
- sodium
- sulfate
- magnesium
- chloride
- potassium
This information provided the foundation for determining specific materials to use to create our hypothetical extreme TDS rinses. Four aqueous solutions were prepared as follows for use during the experiment (see Table 1), with TDS measured and noted using a simple digital TDS tester.
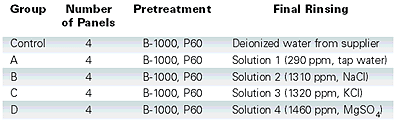 Courtesy of Morton Powder Coatings and Rohm & Haas
Courtesy of Morton Powder Coatings and Rohm & Haas Courtesy of Morton Powder Coatings and Rohm & Haas
Courtesy of Morton Powder Coatings and Rohm & HaasAfter coating application and cure, the test panels were segregated into four sets, two sets of each color. Panels were allowed to dwell for two days in a standard indoor office environment before beginning accelerated corrosion testing.
Test panels were then taped on the reverse, x-scribed and identified prior to corrosion testing. One set of each color was placed on test in a copper accelerated salt spray (GM 4476P) and standard salt spray (ASTM B-117) cabinet. Results were monitored periodically throughout the experiment and are summarized in Table 3.
 Courtesy of Morton Powder Coatings and Rohm & Haas
Courtesy of Morton Powder Coatings and Rohm & HaasResults of Experiment
Only the control group exhibited resistance to accelerated corrosion testing at 312 hours’ exposure, consistent with high-performance end-use applications (see Photo 2). Image Caption
Image CaptionControlling TDS Levels - Measurement
Systematic measurement of source and rinse water TDS levels is crucial. Inexpensive battery operated testers are available from a number of suppliers to test TDS levels. PPM (mg/l) is the most common unit of measure in discussion of TDS. Some testers read in micromhos. Either will work adequately as relative TDS levels and changes are key.TDS readings should be logged at regular intervals throughout the production day. Finishing operators should record pH in the rinse stages. This will provide an early warning of excessive carry over from chemical stages.
Panel Testing
The following test can prove useful in detecting potential coating failures if performed at regular intervals.1. Pretreat, coat and cure a small representative test panel, taking care to ensure the powder coating is applied and cured consistently with the manufacturer’s recommendations.
2. Immerse half of the coated, cured test part in distilled water for 24 hours at ambient temperature.
3. Remove and towel dry the part.
4. After allowing sufficient ambient dwell time to ensure that the coated surface is dry, complete a cross-hatch adhesion tape pull test on both halves of the subject part.
5. Compare results on subject part, and compare to past ‘typical’ performance.
Preventing Problems - De-ionized (DI) Water Rinse
Two reactions produce DI water:91. Metal ions are removed and replaced with hydrogen ions by a cation exchange resin, which is regenerated by an acid.
2. The acid is removed through an anion exchange that is regenerated with an alkaline solution.
DI water has an extremely low TDS level (0–15 ppm) and is the best means of ensuring consistent results. A well-maintained DI final rinse is the best way to remove salts, chlorides and other matter left by previous rinse stages, as well as nonadherent phosphate and other artifacts of pretreatment prior to coating.
Limitations include equipment and operating cost associated with the DI system. Because of DI water’s pure nature, it is corrosive. Stainless steel construction and corrosion resistant components are required in the DI final rinse stage.
Preventing Problems - Reverse Osmosis (RO)
Suspended and dissolved solids are removed by forcing source water through semi-permeable membranes. Typically, RO systems will control TDS levels within the 50–250 ppm range.RO systems are useful when TDS levels in source water are radically higher than RO output TDS. Although not as expensive as DI water, RO treatment requires chemicals to regenerate membranes. RO water can improve coating performance and reduce pretreatment chemical usage when used as make-up water for chemical baths. However, DI water is preferable as a final rinse, because the typical purity of RO water falls short of levels required for best coating performance.
Mechanical Control
If source water purity is adequate to produce the desired coating corrosion resistance, overflowing or counterflowing of rinse stages can prove both an effective and less costly means of controlling rinse water TDS. In these systems, source water is introduced to the final rinse stage at a rate sufficient to maintain TDS at an acceptable level (typically 10–40 liters per minute). Water is conserved by overflowing or backflowing final rinse water into earlier rinse stages. Final rinse TDS should be controlled to a maximum of 1.5 times source water TDS. Similarly, first rinse TDS should not exceed four times source water TDS and second rinse no more than three times source.10Potential variability in source water TDS levels, and rinse contamination from chemical carry over resulting from part mix and production rate makes TDS monitoring crucial with this approach. Furthermore, when typical production final rinse levels routinely exceed 100 ppm, coating performance testing should be performed to ensure that the desired resistance properties are realized.
Conclusion
Rinse water quality influences coating performance necessitating vigilant monitoring, treatment and control to ensure corrosion performance. DI water final rinse is a necessity for high performance applications.Metal pretreatment differs from coating application in one important way. Problems, particularly with rinse water, are seldom seen in a casual inspection. Careful monitoring and testing is vital to determine the ongoing effectiveness of part rinsing. If TDS levels are not controlled, coating sheet delamination, blistering and other costly coating failures can and will result.
This paper was presented at Powder Coating Europe 2000 in Amsterdam, The Netherlands.
For more information on the effect of rinse water quality, contact Thomas P. Frauman, Marketing Manager, Morton Powder Coatings, 3 Commerce Drive, Reading, PA 19607; e-mail tfrauman@rohmhaas.com.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!







