In Part 1 of this article series,1 we discussed a new class of reactive surfactants, both nonionic and anionic, for use in both conventional aqueous emulsion polymers and in UV-curable coatings that contain two polymerizable moieties. These new reactive surfactants can be employed either as emulsifiers or as pigment dispersants.
Our new reactive surfactants are block copolymers of two or more moles of allyl glycidyl ether (AGE) and ethylene oxide terminated on the AGE end with a hydrophobic moiety. The addition of a second equivalent of AGE significantly reduces the amount of non-reactive surfactant in the product so that increases in coating repellency may be achieved.
These new reactive surfactants may contain various hydrophobes such as styrenated phenol ethers, alkyl ethers such as tridecyl alcohol, or alkylphenol ethers. Varying the level of ethoxylation and adjusting the size of the hydrophobe results in being able to tune the structure for optimal performance for each application.
These nonionic copolymers may be further derivatized to convert them to anionic surfactants. Examples of these include sulfonates, phosphate esters and carboxylates.
Following are two further studies designed to elucidate the benefits of reactive surfactants containing more than one reactive group rather than a single reactive group. To avoid differences based on reactive group differences, the allyl group is the reactive moiety in all of the reactive surfactants.
These new surfactants were used to prepare wood deck stains and direct-to-metal coatings. Their properties were then compared to coatings based on latexes prepared with mono-functional surfactants. In addition to learning about the number of reactive groups, some discoveries about hydrophobe type and level of ethoxylation (hydrophile size) are discussed.
Study 3: Formulated Deck Stain Testing
In the previous studies, a latex formula was developed that allowed for the property differentiation of specific polymerizable surfactants. Various tests were performed to elucidate the properties imparted to the latex by the surfactants. Conclusions indicated that ERS 01618 (E-Sperse® RS-1618) produced the most hydrophobic latex. Data is reported here elucidating that the ERS 01618 surfactant imparts excellent water resistance to the final formulated deck stain coatings. Water beading confirmed that ERS 01618 produced superior hydrophobicity compared to other surfactants.
Experimental Procedure for Deck Stain Formulation
Materials
The four latexes in Table 1 were formulated into deck stains. The formulation procedure for the deck stain is shown in Table 2. The ingredients were added, in the order listed, to a stainless steel beaker under agitation. The paint was mixed for a further 15 min and stored in ½-pint cans. Paints were made with Aqua Bead 525 using latexes made from NPES-930 and ERS 1618 in order to determine the effect of this wax dispersion on water beading properties as a function of surfactant type.
Surfactant Type Effects on Water Beading
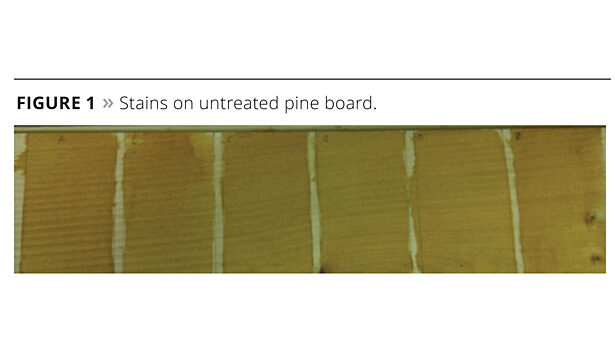
Deck stain performance is often demonstrated through its ability to produce water beads when sprayed with water. Latexes that are more hydrophobic in nature provide superior water beading. To determine the water beading character of the latexes that were formulated into deck stains, the stains were applied to a southern yellow pine board. Three grams of stain were applied to a 3" x 5" area of the wood and dried overnight (Figure 1).
The next day a drop of water was placed on top of each board, a photograph taken, and contact angle measured. The results are shown in Figure 2.
The numerical results for the contact angle measurements are listed in Table 3. The contact angle of NPES-930 is the lowest. This is expected since the surfactant is not bound to the latex and is free to migrate, lowering the surface tension of the drop. The other surfactants demonstrate significantly higher contact angles, with ERS 01618 having the largest. Upon addition of the Aqua Bead, the NPES-930 contact angle significantly increased, but not to the extent of the two best surfactants, BC-1025 and 1618, without Aqua Bead. This demonstrates the excellent water beading characteristics that polymerizable surfactants can impart to the latex. This may allow for less wax additives in the formula improving durability.
The ERS 01596 surfactant with one reactive group did not perform as well as ERS 01618, which has a similar structure, but two reactive groups, nor as well as the Hitenol nonylphenol-based reactive surfactant. ERS 01596 contains a higher fraction of non-reactive free surfactant than ERS 01618, which may have led to its lesser repellency, and has a much longer EO chain than the Hitenol, thus making it more water sensitive.
Stain Odor
A simple odor test was performed on the final stains. The stains were placed into ½-pint cans and allowed to sit for 1 h. The headspace was sampled and relative odor assessed. The results are shown below from strongest to weakest:
NPES-930 >> BC-1025 > 01596 = 01618
Latex stains containing Ethox surfactants have the lowest odor. Presumably, the reactive surfactants have largely reacted into the latex and thus cannot volatilize into the headspace.
Conclusions from Study 3
Contact angle measurement of water beads on the formulated deck stain indicated superior water beading properties of 01618 over other surfactants in this study. Presumably, its higher functionality provided less non-reactive surfactant to reduce the surface tension.
Study 4: Comparison of Difunctional Polymerizable Surfactant with Non-Reactive Products
The purpose of our fourth study was to develop styrene acrylic latexes and paint formulations for metal applications that allow for the differentiation of polymerizable surfactants and comparison to currently available and common non-polymerizable surfactants.
Materials
The surfactants used in the study were ERS 01618 – POE(15) {styrenated phenol /2 AGE} sulfate, from Ethox, and E-Sperse 704 – POE (20) styrenated phenol sulfate, from Ethox.
Experimental Procedure for Latex Synthesis
All latexes were produced in a jacketed glass reactor. Temperature was regulated by an external circulating water heater. The reactor was fitted with a thermocouple, reflux condenser and feed lines. The monomer was fed subsurface through a stainless steel tube. The initiator (oxidizer and reducer) was added above the surface of the latex. Both monomer and initiator were fed via FMI pumps (Table 4).
Experimental Procedure for Paint Formulation
The ingredients were added, in the order listed, to a stainless steel beaker using a high-speed disperser (Table 5). A good grind was obtained. Next, the letdown was made using low speed on the same disperser. The paint was mixed for a further 15 min and stored in ½-pint cans.
Results and Discussion
Particle size
The polymerizable surfactants produced smaller particle sizes than the non-polymerizable surfactants (Table 6). Smaller particle sizes were also obtained with higher-Tg latexes. It is not readily apparent why this is the case. Note that the monomer ratio for the low-Tg latex is richer in butyl acrylate than methyl methacrylate and, therefore, is more hydrophobic. Theory predicts smaller particles with more hydrophobic latexes as the surfactants absorb more strongly to the surface.
Reactor Fouling
Reactor fouling was worse for the high-Tg latex than for the low-Tg latex. This may be due to the smaller particle size obtained with the high-Tg latex. Reactor fouling is worse with polymerizable surfactants in both low- and high-Tg latexes.
Latex Stability
The stability of the latexes was obtained indirectly by measuring the filterable solids in the latex (Table 7). The formula containing only polymerizable surfactant in the low-Tg latexes (147) produced the highest 40-mesh filterable solids. This corresponds well with the level of reactor cleanliness, or lack thereof in this case. The 150-mesh filterables were essentially the same. Formula 147 was less foamy than 146 in the shear test. This is expected as free surfactant normally creates more foam.
The high-Tg latexes had lower filterable solids. Recall, however, that the reactor was much more fouled as compared to the low-Tg latexes. The latex with free surfactant only (148) failed the high-shear test after 6 min. The test runs for 10 min. The lowest foaming latex in the shear test was formula 149, the only latex that had no free surfactant. It also passed the test while having the smallest particle size.
The pre-emulsions were easily formed and were stable in all cases. In-process foaming was low. All monomers were easily reduced to less than 150 ppm.
Filterable solids are higher than what would be typical for production; however, it does show differences in latex performance. Styrene acrylics are more difficult to stabilize than other types of latex polymers. Note that the 40-mesh filterable solids can include some reactor wall material. It is best to use the 150-mesh results since this reflects the filterable solids with the 40-mesh material already removed.
The shear stability tests confirm that polymerizable surfactants improve high shear stability and reduce foaming. After shearing in the Hamilton Beach blender at medium speed for 10 min, the ERS 01618-containing latexes were less foamy. This was expected since free surfactants contribute to shear-induced foam. Note that the 149 latex, with non-polymerizable surfactants only, failed the shear test after 6 min.
Foaming in the shake test did not show significant variation. The 147 formula was slightly worse than the others were. Note that the foam here is different from the foam in the shear test. These bubbles were large while those in the shear test were very small. Styrene acrylics foam more than other latexes in general. Much of the foam is generated by the persulfate ended styrene-containing oligomers formed in the latex polymerization procedure.
The latexes did not exhibit freeze thaw resistance.
Dry Film Characteristics
Table 8 indicates the resistance to water whitening of the dried latex films. Since the higher-Tg latexes (148 and 149) will not form a film at room temperature, 18% UCAR Filmer IBT on solids was added to the latex to facilitate coalescing.
Polymerizable surfactants improve blush resistance. This is demonstrated in Figure 3 for the low-Tg latexes. The high-Tg polymers were also tested and showed no blushing at all over a period of 3 h. High-Tg latexes, which are softened with solvent, regain their hardness over time. This hardness resists water ingress and significantly reduces blushing. It is expected that blush resistance will grow in importance as solvents are taken out of coating systems and latexes will have to be made softer.
Effect of Surfactants on Formulated Metal Coatings
Paint Formulation Results
The paints were drawn down on Leneta charts at 4 mil and 10 mil wet to a final dry thickness of about 2 and 5 mils respectively (Figure 4) and dried for 16 h at ambient temperature. The paint films of the high-Tg latexes are shown, and are similar to the low-Tg paint films. The 2 mil dry film showed cratering for the latex made with ERS 01618 while the 5 mil films did not. Note also that these same paints had reduced gloss and hiding. It is possible that the difunctional surfactant is crosslinking the latex during manufacture, not allowing the uptake of solvent into the particle, which in turn causes cratering and low gloss. Lower hiding often results from poorer TiO2 dispersion in the paint. It is possible that the more hydrophobic polymerizable surfactant-containing latex robs some of the stabilizing moieties from the TiO2 grind, causing flocculation and reduced hiding. This flocculation may also account for the lower gloss.
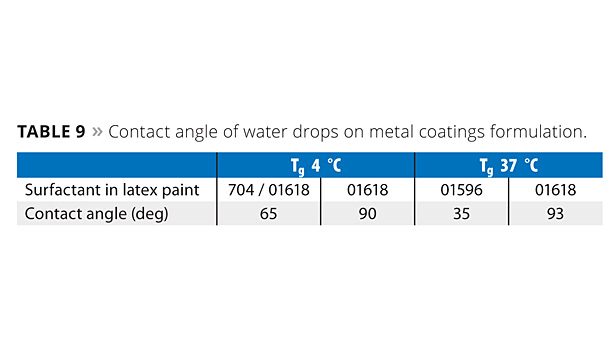
Effect of Surfactant Type on Coating Hydrophobicity
The exclusion of water from the coating/metal substrate interface helps to control corrosion. This in turn reduces the chance of delamination from the substrate due to osmotic pressure built up by contaminants at the interface. Figure 5 demonstrates the hydrophobicity of the metal coatings by examining the contact angle of a drop of water on the coatings. Higher contact angles are usually indicative of more hydrophobic coatings. The contact angles are given in Table 9. The highest contact angles are achieved with the difunctional reactive surfactant ERS 01618.
Blister Resistance
Blisters are formed due to migration of water to the coating/substrate interface. Osmotic pressure (due to the dilution of water-soluble species at this interface) builds, causing a blister to form in coatings that lose adhesion to the substrate and expand. Lower-Tg coatings are more susceptible to this. Free surfactants at the interface also exacerbate this problem. Both of these situations are encountered in low-VOC coatings as well as those containing free surfactants. Figure 6 demonstrates this. Blistering occurs to a greater extent in the coating that contains the E-Sperse 704. The lower-Tg coatings also form more blisters than their higher-Tg counterparts. This blistering problem will become a greater issue in low-VOC coatings due to their inherently lower modulus.
Conclusions from Study 4
• Particle size is smaller for latexes when polymerizable surfactants are used.
• Reactor fouling is worse with polymerizable surfactants
• The polymerizable surfactants produced shear-stable products in the blender shear test.
• All surfactants produced good, stable pre-emulsions. The pre-emulsions were easy to make (added monomers under agitation, adding the most hydrophobic monomers first).
• Polymerizable surfactants had lower blush than non-polymerizable surfactants in the low-Tg latexes. Presumably, this is due to improved hydrophobicity and less free surfactant present.
• Paints made with latexes containing polymerizable surfactants had lower gloss and poorer hiding. They were also prone to cratering. This could be due to some crosslinking of the latex with concomitant reduction of ability to flow and coalesce.
• Paints made with latexes containing polymerizable surfactants had improved hydrophobicity and lower tendency to blister.
Overall Conclusions from Studies 1-4
The performance of ERS 1618 shows that adding a second reactive group improves the hydrophobicity of latexes prepared with reactive surfactants.
• ERS 1618 surfactant-based latexes appear to have good blister resistance, supporting the theory that free surfactant leads to blistering and poor adhesion problems.
• Reactive surfactants tend to cause more reactor fouling.
• Paints with reactive surfactants appear to have worse hiding power and lower gloss in the formulations examined. Work to correct this effect has been conducted, and hiding power and gloss have actually been improved over non-reactive surfactants. This will be reported in a future study.
• Styrenated phenol-based reactive surfactants appear to outperform tridecyl alcohol-based reactive surfactants.
• Further work to explore the performance of these reactive surfactants is necessary to better understand how to maintain the good properties they provide while minimizing the negatives. n
For more information, visit www.ethox.com.
References
1 Palmer, Charles F. New Reactive Surfactants for Emulsion Polymerization, Part 1, Paint & Coatings IndustryMagazine, Oct. 2012, p. 38.

















Report Abusive Comment