New Reactive Surfactants for Emulsion Polymerization, Part 1




Like non-reactive surfactants, reactive surfactants are molecules that typically have a hydrophobic segment and an ionizable and/or polar group. The hydrophobic segment preferentially adsorbs onto the surface of the latex particle during and following particle polymerization. The hydrophilic group extends into the aqueous solution phase and provides a steric barrier or charge repulsion against particle agglomeration and coagulation.
Unlike their non-reactive counterparts, reactive surfactants additionally contain a reactive group on the hydrophobic segment that is capable of covalently bonding to the latex surface. Usually this is a moiety such as a meth(acrylate) ester or a terminal olefin that can participate in free-radical emulsion polymerization reactions. When used in an emulsion polymerization a large fraction of the surfactant molecules become irreversibly bound to the emulsion polymer chains and droplets.2,3 This can improve both the latex stability and reduce foaming.
A number of coating properties are typically improved as well, especially water resistance. Once a latex-containing product has been applied to a surface as part of a coating, the surfactant is no longer needed. In fact, the presence of the surfactant often degrades the moisture sensitivity of the coating. Other coating properties can be negatively affected as well. This is largely due to the mobility of the surfactant polymers. For example, locally high concentrations of surfactant molecules can form in the coating from the coalescence of surfactant-coated micelles. When the coating is exposed to water, these unbound surfactant molecules can be extracted from the coating, leaving thin spots or pathways to the substrate surface. This can result in a pinholing effect and substrate corrosion.4
A number of reactive nonionic and anionic surfactants are commercially available. Most of these are designed to participate in free-radical polymerizations. However, none of these reactive surfactants incorporate more than one reactive moiety in their structure.
We are developing a new class of reactive surfactants, both nonionic and anionic, for use in both conventional aqueous emulsion polymers and in UV-curable coatings that contain two polymerizable moieties. These new reactive surfactants can be employed either as emulsifiers or as pigment dispersants.
The patent literature discloses reactive surfactants (US 4,814,514) prepared by the addition of allyl glycidyl ether (AGE) to surfactant base hydrophobes such as hydroxyl-functional fatty alcohols or substituted phenols. However, the molar ratio of AGE to hydrophobe is 1:1. Due to the nature of epoxide reactions with hydroxyl functional materials, a distribution of adducts will be formed. Some of the hydroxyls will react with AGE as desired to make the 1:1 adduct, while some of these 1:1 adduct products will add a second mole of AGE to make the 2:1 adduct.
For every mole of the 2:1 adduct formed, one mole of unreacted hydroxyl functional material will remain. This material will continue through the second-step reaction with ethylene oxide (EO) to form a non-reactive surfactant. In addition to the undesired 2:1 adduct formed, some 3:1 adduct will be formed as well. For every mole of 3:1 adduct, two moles of surfactant base is left unreacted.
Our new reactive surfactants are block copolymers of two or more moles of allyl glycidyl ether (AGE) and ethylene oxide terminated on the AGE end with a hydrophobic moiety. The addition of a second equivalent of AGE significantly reduces the amount of non-reactive surfactant in the product so that increases in coating repellency may be achieved.
These new reactive surfactants may contain various hydrophobes such as styrenated phenol ethers, alkyl ethers such as tridecyl alcohol, or alkylphenol ethers. Varying the level of ethoxylation and adjusting the size of the hydrophobe results in being able to tune the structure for optimal performance for each application.
These nonionic copolymers may be further derivatized to convert them to anionic surfactants. Examples of these include sulfonates, phosphate esters and carboxylates.
Generally, latex particles can be prepared by mixing monomers together to form a monomer mixture. A surfactant (or surfactants) is (are) then added to the monomer mixture and sheared into water to form an emulsion. The surfactant(s) may include a reactive surfactant, a nonreactive surfactant, or a combination of reactive and nonreactive surfactants.
A covalent bond-forming reaction occurs between the reactive surfactant and the latex particle surface. The “locking-in” of the surfactant onto the surface of the latex particle results in the improved properties observed with the use of reactive surfactants. Some of the improved properties include dispersion stability of the latex under high thermal shear conditions and improved water repellency and water resistance of coatings based on the latex. Presumably, these latter two are a result of less free surfactant in the coating, as described above.
Following are two studies designed to elucidate the benefits of reactive surfactants containing more than one reactive group rather than a single reactive group. (Part 2 of this article will report on two further case studies.) To avoid differences based on reactive group differences, the allyl group is the reactive moiety in all of the reactive surfactants.
These new surfactants were used to prepare wood deck stains and direct-to-metal coatings. Their properties were then compared to coatings based on latexes prepared with mono-functional surfactants. In addition to learning about the number of reactive groups, some discoveries about hydrophobe type and level of ethoxylation (hydrophile size) are discussed.
Study 1: Deck Stain Formulations With Reactive Surfactants.
The first study compared ERS 01596 and ERS 01618 with competitive commercial products in latexes for deck stain applications.
Experimental
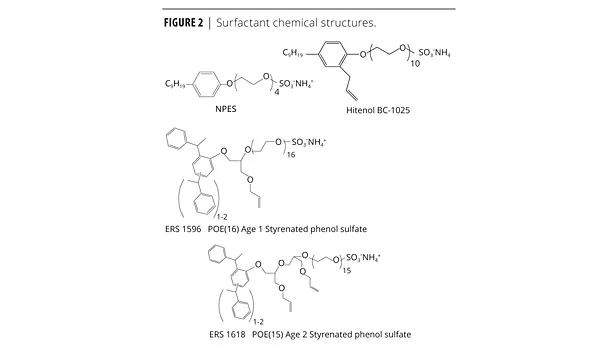
Surfactants used in this study include the following; their chemical structures are shown in Figure 2.
• Aerosol NPES – 930, POE 4 (polyoxyethylene) nonylphenol (NP) ammonium sulfate – Cytec.
• Hitenol BC-1025, believed to be POE 10 allyl-substituted nonylphenol – Montello.
• ERS 1596 (E-Sperse® RS-1596) is a reactive surfactant based on a styrenated phenol hydrophobe with one equivalent of allyl glycidyl ether, then ethoxylated with 16 moles of EO, sulfated, and neutralized – Ethox Chemicals.
• ERS 1618 (E-Sperse® RS-1618) is a reactive surfactant based on the same styrenated phenol hydrophobe as ERS 1596 with two equivalents of allyl glycidyl ether, then ethoxylated with 15 moles of EO, sulfated, and neutralized – Ethox Chemicals.
The AGE-based reactive surfactants were prepared by the sequential reaction of AGE with the hydrophobe listed, followed by further reaction with ethylene oxide. Sulfates were prepared by reaction of the ethoxylates with sulfamic acid and were used in the ammonium salt form. Commercial surfactants were purchased from the manufacturers listed and used as is.
Latex Formulas
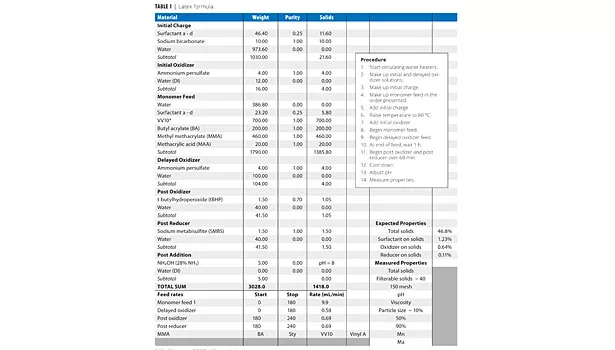
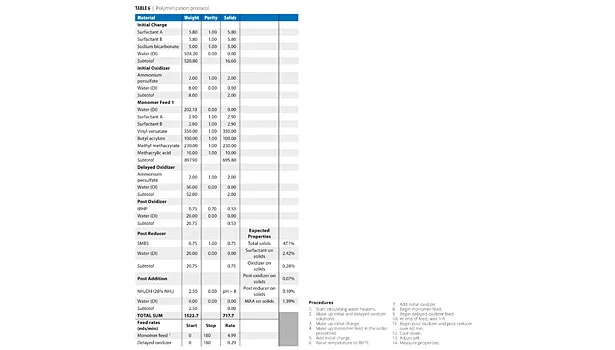
All latexes were produced in a jacketed glass reactor; temperature was regulated by an external circulating water heater. Table 1 shows the basic formula that was used. The reactor was fitted with a thermocouple and feed lines. Monomer was fed subsurface through a stainless steel tube. Initiators (oxidizer and reducer) were added above the surface of the latex. Both monomer and initiator were fed via FMI pumps.
Results and Observations
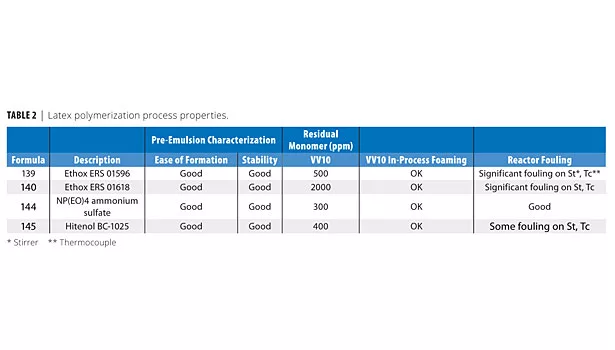
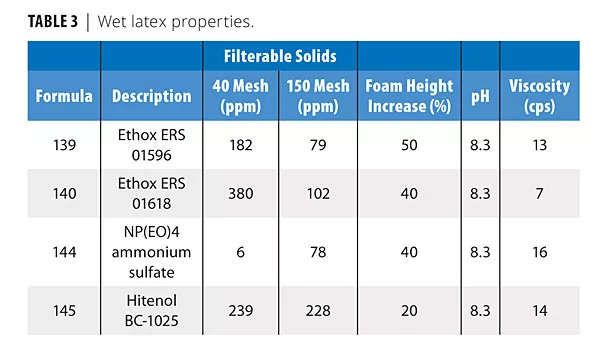
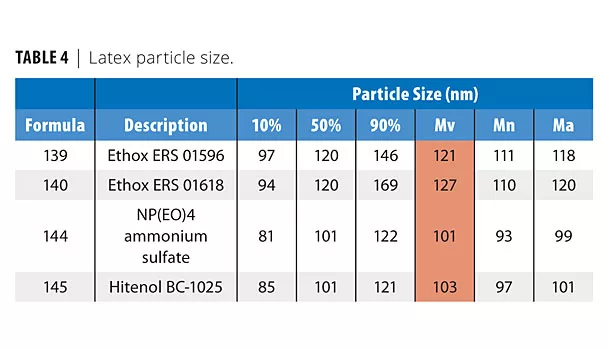
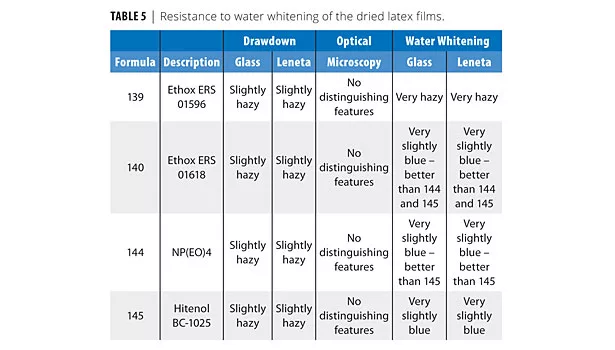
As Table 2 shows, all surfactants produced good shear-stable products. Table 3 shows that all surfactants produced very low filterable solids, and that foaming was variable in the shake test, with Hitenol BC-1025 producing the lowest foam (shake 50 g of latex in 100 mL graduated cylinder 20X). Table 4 shows that the two DSP-based surfactants produced larger particle sizes than NP(EO)4 ammonium sulfate or Hitenol BC-1025. Table 5 shows that ERS 01618 had the best blush resistance of the four surfactants studied, and ERS 01596 had the poorest blush-resistant properties.
Study 1 Conclusions
• All surfactants produced very low filterable solids.
• Foaming was variable in the shake test, with Hitenol BC-1025 producing the lowest foam. It is believed that this is due to the higher level of ethoxylation on the styrenated phenol surfactants ERS 01596 and ERS 01618 (15-16 moles of EO) than on the BC-1025 surfactant (10 moles of EO). Higher levels of ethoxylation are known to increase foaming tendency. Styrenated phenol surfactants are known to be generally low foaming.
• DSP-based surfactants produced larger particle sizes than NP(EO)4 ammonium sulfate or Hitenol BC-1025. It is believed that this is also due to the higher level of ethoxylation on ERS 01596 and ERS 1618. (See explanation below.)
• All surfactants produced good shear-stable products.
• ERS 01618 had the best blush resistance of the four surfactants studied.
• ERS 01596 had the poorest blush resistant properties. We are not sure why this would be the case, but it could be because of the long EO chain and a high fraction of non-reactive surfactant in the product.
The larger surfactants with more ethoxylation have lower charge density than the smaller NP(EO)4 or Hitenol products and thus less charge stabilization. This would account for the larger particle size. Long EO chains stabilize foam as well. Presumably, similar DSP-based surfactants with a lower level of ethoxylation would perform better in both particle size and foaming. The surfactant with two reactive groups (ERS 01618) significantly outperformed the similar surfactant with one reactive group (ERS 01596) in blush resistance, a good measure of water repellency.
Study 2: Further Study of Deck Stain Formulations with Reactive Surfactants
Materials
The surfactants used in this study were as follows:
• ERS 01618 - POE(15) (styrenated phenol/2 AGE) Sulfate – Ethox
• ERS 01617 (E-Sperse® RS-1617) - POE(15) (styrenatedphenol/2 AGE) – Ethox
• ERS 01651 - POE(15) (tridecyl alcohol(TDA)/1 AGE) Sulfate – Ethox
• Aerosol NPES – 930 - NP(EO)4 ammonium sulfate – Cytec
• Hitenol BC-1025 - POE(10) (NP /1 allyl) Sulfate – Montello
• Hitenol KH-1025 - POE(10) (TDA /1 allyl) Sulfate – Montello
TDA is tridecyl alcohol; NP is nonylphenol
Latex Synthesis Procedure
All latexes were produced in a jacketed glass reactor; temperature was regulated by an external circulating water heater. The reactor was fitted with a thermocouple, reflux condenser and feed lines. Monomer was fed subsurface through a stainless steel tube. Initiators (oxidizer and reducer) were added above the surface of the latex. Both monomer and initiator were fed via FMI pumps. The formula is shown in Table 6.
Note that the surfactants are 1.24% on solids. The reaction shown in Table 6 indicates 2.42% on solids. This is due to the additional use of the nonionic surfactant ERS 01617 in that formula, doubling the total concentration of surfactant.
Reactions
Table 7 outlines the experiments from Study 1 in green (Formulas 139, 140, 144 and 145) and those produced for Study 2 (150, 151, 152, and 169).
• Formula 150 was run to add statistical relevance (150 compared to 140).
• Formula 151 was used to determine the effect of addition of nonionic polymerizable surfactants.
• Formula 152 and 169 compare the Ethox TDA surfactant ERS 01651 to the TDA-based Hitenol KH 1025.
Effect of Polymerizable Surfactants on Particle Size
Recall that in Study 1, the polymerizable surfactants ERS 01596 and ERS 01618 produced 20% larger particle sizes than other surfactants based on Mv (see Table 7, data highlighted in green). The ERS 01618 experiment (140) was repeated (150) to add some statistical relevance to the data. Note that the particle size for 150 is similar to 140, indicating that the styrenated phenol surfactants definitely do produce slightly larger particle sizes.
A second experiment was run using ERS 01617 in addition to ERS 01618 in equal concentrations, both in the initial charge and the pre-emulsion (151). The particle size increased dramatically. Note that nonionics normally cannot be used as the sole surfactant because the latex will not be stable.
Finally, the two TDA-based surfactants (Formulas 152 and 169) were compared. The Hitenol KH 1025 surfactant produced smaller particles than the ERS 01651. Possible reasons include the level of sulfonation and charge per gram of surfactant as discussed earlier. Note that the particle size distribution for ERS 01651 (169) had a small peak at about 1µm, indicating some instability during polymerization.
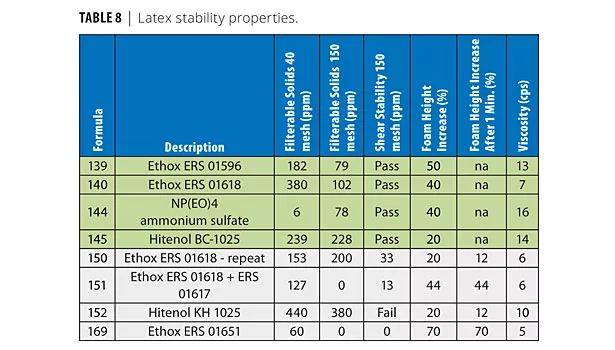
Latex Stability
Reactor Filterable Solids
The filterable solids from the latex reactions are shown in Table 8. The data from the prior reactions (139, 140, 144 and 145) highlighted in green, are included in this report for completeness and ease of comparison. The cleanest latex contains 01651 (169).
Shear Test and Resulting Filterable Solids
The filterable solids from the shear test are also shown in Table 8. The data from the previous study highlighted in green (139, 140, 144 and 145) are included in this report for completeness and ease of comparison. Only pass/fail data are noted for this previous set of data. The cleanest latex contains 01651 (169). Note that the particle size for 01651 is somewhat larger, so greater stability in the shear test is anticipated. This may also be the case for reaction 151 with the added nonionics (011596). The Hitenol KH does not impart as much stability as the ERS 01618 or ERS 01651 surfactant, as indicated by the failure in this test.
Test
The graduated cylinder shake foam test indicates that 01651 produced the most foam. It is not readily apparent why this is the case.
Latex Viscosity
Latex viscosities are similar. This is expected as the viscosity is low, thus reducing any particle size or stability impact on viscosity.
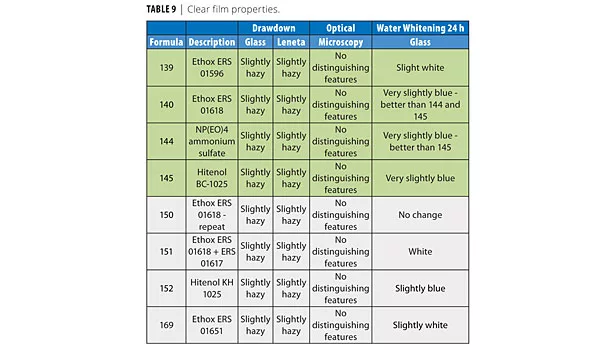
Clear Film Characterization
Optical Characteristics
The latexes were drawn down on glass to produce films of 5 mil thickness. All the films looked similar and were slightly hazy (Table 9). The data for reactions from the previous study (139, 140, 144, and 145) are included for completeness and ease of comparison.
Water Spot Resistance
The films were dried for 24 h and subjected to a 24 h water spot test. The ERS 01618 was the best for water whitening. The latex containing nonionics was the worst (151). This is expected since there was twice as much surfactant in this latex. The two TDA surfactants, ERS 01651 and KH 1025, were worse than their aromatic-based counterparts (01618 and BC-1025 respectively).
Water Soak Test Results
Figure 3 shows the trends for the water spot tests (over glass) for surfactants used in the previous study.
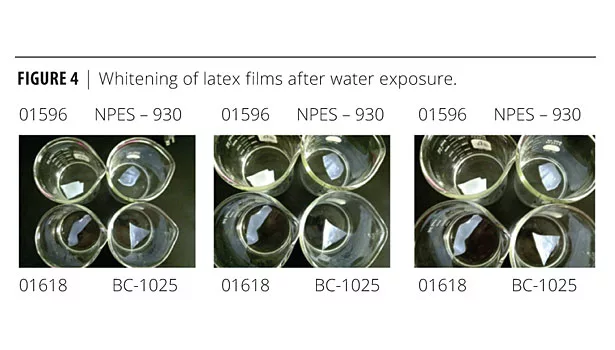
A similar study was performed by soaking dried samples in water. The samples were cast over polyethylene sheeting and allowed to dry for 4 days, turning the samples over each day to aid in the drying process. The final sample thickness was 1/8 inch. These samples were then placed in 600 mL beakers and covered with 200 mL of water. Water uptake, as measured by change in opacity, was noted as a function of time (1 day, 2 days and 4 days). Whiter films indicate higher water sensitivity with these latexes. The data is presented in Figure 4.
No further changes were noted after the 4 days of soaking, even after 2 weeks. The order of water whitening (water sensitivity) is as follows and correlates well with the water spot test:
Most whitening 01596 > BC-1025 > NPES0 930 > 01618 Least
Heat Age Stability
Samples of the latex made with the various surfactants in Study 1 Part A (139, 140, 144, and 145) were placed in polycarbonate jars. The jars were capped with a screw top lid. They were then placed in a 50 °C (+/- 1 °C) oven for 1 month. The samples were examined weekly to determine if there was any settling. Pictures of the samples initially (Figure 5) and after one month (Figure 6) are shown.
The heat-aged samples were removed from the oven after 1 month and cooled to room temperature. A clear water layer was not observed on the top of the latex, indicating no visual settling.
Freeze-Thaw Resistance
The latexes were placed in a ½-pint metal paint container. They were frozen overnight at -5 °C. Freeze-thaw tests indicate that all surfactants used in this study did not pass one freeze-thaw cycle at the concentrations employed. This is not unexpected as it is typically nonionic surfactants that impart freeze thaw properties.
Recall that in experiment 151, ERS 01617 was employed in addition to ERS 01618, doubling the concentration of surfactant. Nonionic surfactants are thought to impart freeze-thaw characteristics. Upon thawing, there was significant coagulation, however not as much as that with 01618 alone. Improved freeze-thaw could be expected if higher MW polyether on the surfactant molecule and higher concentrations of the polymerizable surfactant were employed in the latex.
Conclusions From Study 2
• It was confirmed that the type of surfactant affects particle size with the styrenated phenol-based surfactants producing particle sizes at least 20% larger than the other in the study when employed with VV10 type latexes.
• The shear stability for the two TDA-based polymerizable surfactants indicates that ERS 01651 is the most stable in the finished product. Note that it had the largest particle size, so increased stability in the final product is more easily obtained.
• ERS 01651 produced the most foam in the shake foam test.
• The best water resistance of all surfactants studied was obtained with ERS 1618. Presumably, the higher functionality of ERS 1618 results in less non-reactive surfactant to reduce the surface tension.
• ERS 1618 has the best blush resistance of the four surfactants studied in the water immersion test. Presumably, its higher functionality results in less non-reactive surfactant that can cause coating defects.
• Heat age tests at 50 °C for 1 month indicate that all surfactants (NPES0-930, BC-1025, ERS 01596 and ERS 016180) produced heat-aged stable products. n
For more information, e-mail cpalmer@ethox.com.
References
1 Wikipedia, “Emulsion Polymerization”
2 Huang H.; Lu D.; Shen L.; Guan R. Reactive Surfactant in the Emulsion Copolymerization of Methyl Methacrylate and Octyl Acrylate, Journal of Macromolecular Science, Part A: Pure and Applied Chemistry 2008, 45, 242-247.
3 Brazanga-Pugh S. Role of Reactive Surfactants in Miniemulsion Polymerization, Ph.D. Thesis, Lehigh University, 2010.
4 For a review, see Guyot A. and Tauer K., Reactive Surfactants in Emulsion Polymerization, In: Advances in Polymer Science, Vol. III, Springer-Verlag, Berlin, 1994, 43-65.
This paper was presented at the 39th Annual Waterborne, High-Solids and Powder Coatings Symposium in New Orleans.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!





