Zinc-Rich Primers
Sacrificial and/or Barrier?

Zinc-rich primers (ZRPs) have been a major anticorrosion technology in protective markets for decades.1 Industry specification bodies and customers largely adopted specifications based on zinc percentage. This article highlights recent market innovations and emerging technologies that challenge the traditional belief that higher zinc content equates to better corrosion resistance.
ZRP Background
According to a report from the National Association of Corrosion Engineers (NACE), which is now the Association of Materials Protection and Performance (AMPP), the global economic impact of corrosion is estimated to be US $2.5 trillion.2 Protective coatings largely address this issue by significantly increasing the lifetime of substrates. For example, protective coatings on cars or bridges can extend their life over decades. This is a vast area of research, and coatings suppliers offer a variety of solutions ranging from pretreatments, primers, basecoats and clearcoats, but mechanistically they can be classified into barrier and sacrificial protective coatings. End users select protective coatings based on the environmental conditions, specifications, life span expectations, application parameters and, sometimes, regulatory requirements.
Barrier protective coatings (BPC) utilize crosslinking networks and adhesion to achieve a nonporous film that delays external elements from reaching the metal substrate (Figure 1). Often, epoxy has been the chemistry of choice for barrier coatings, with some exceptions. However, when there is physical damage to the barrier coating and it is exposed to external elements, the substrate can corrode.

Sacrificial protective coatings (SPC) safeguard steel surfaces using more electrochemically active metal pigments, such as zinc, which acts as a sacrificial anode and preferentially corrodes instead of the steel substrate, which acts as a cathode. Unlike BPC, SPC is not impacted by minor film damage, as the sacrificial components “self-heal” to protect the substrate. Zinc has been primarily used as the sacrificial anode material, and products are sold as zinc-rich primers under various brand or trade names (Figure 2).
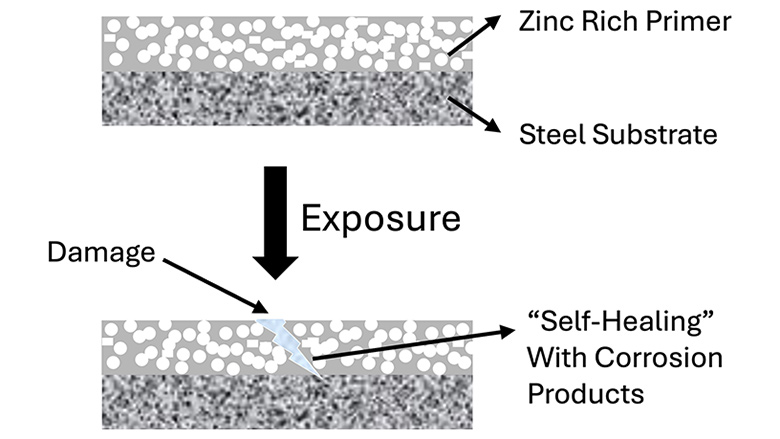
It is interesting to note that although ZRPs are often described as sacrificial, both barrier and sacrificial mechanisms work together to prevent steel corrosion, especially epoxy ZRPs. As highlighted in the “Lower Zinc ZRPs” section below, there is immense potential to optimize barrier and sacrificial mechanisms to achieve optimal levels of corrosion resistance.
Specifications and the Need for Innovations
As the SPC protection mechanism is sacrificial or cathodic protection, the extent of protection has generally been expected to depend on the level of sacrificial elements. There are standards and specifications defining the percentage of zinc. Specifically, the Society for Protective Coatings (SSPC), now part of the AMPP, Paint Specification 20 describes two types of zinc-rich coatings with three zinc levels in the dry film:
- Level 1 ≥ 85%
- Level 2 is ≥ 77% and < 85%
- Level 3 is ≥ 65% and < 77%
The industry generally follows the principle that a higher zinc content leads to better corrosion resistance. Customers rely on standards such as ISO 12944, NTPEP and SSPC Paint 29 that stipulate weight percentage of zinc.1
However, as listed below, high-zinc ZRP formulations present several application and sustainability challenges:
- Films are brittle; any substrate movements can lead to cracks3
- Solvent trapping
- Poor transfer efficiency and difficulty in building film thickness
- Add weight to the infrastructure
- Need topcoats and/or tie coats to manage sacrificial nature, along with UV durability and aesthetics
These challenges make high-zinc ZRPs difficult to use. Additionally, zinc ranks as the fourth most widely used material, behind iron, aluminum and copper, with approximately 20 million tons produced annually. This element has been used in a range of applications from coatings to skin care products. Prolonged exposure to zinc can have health hazards, as shown in these products’ safety data sheets. The cost of zinc has also impacted markets negatively. Given zinc’s broad usage, supply chain constraints, potential hazards and application issues, it is imperative that the coatings industry explores maximizing zinc performance while lowering the levels used in coatings.
Lower-Zinc ZRPs
In recent years, progress has been made toward lowering the zinc content in ZRPs, offering different perspectives on barrier versus sacrificial aspects. A recent study4 highlighted the role of zinc but also other factors such as polymer chemistry, crosslink density, pigment/binder ratio, etc., and concluded that the corrosion resistance of ZRPs is not solely dependent on the percentage of zinc in the coating but rather several other factors.
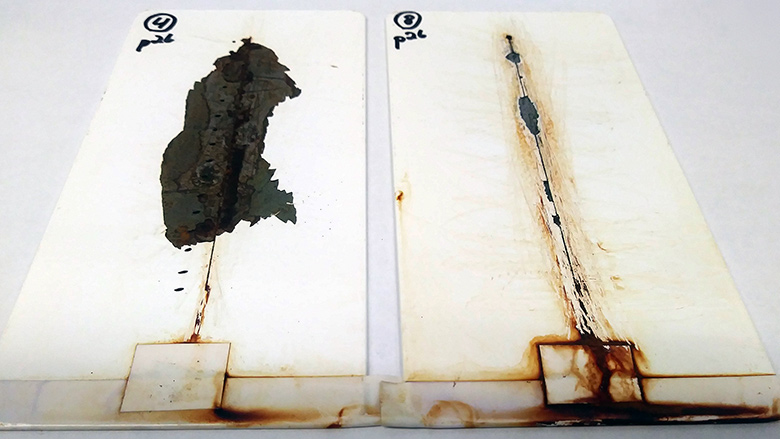
Several research articles have highlighted the use of conductive materials such as carbon nanotubes,5 graphite,6 polyanilines and polypyrroles7 to reduce the zinc levels and/or improve corrosion performance (Figure 3). In addition, ZRPs with glass spheres and other proprietary materials surpassed traditional ZRPs in corrosion resistance and mechanical properties.8

Recently launched PPG PRIMERON™ optimal zinc epoxy powder primer offers optimized zinc amounts, much lower than traditional ZRPs, to deliver exceptional transfer efficiency, scribe corrosion resistance and excellent adhesion on multiple substrates (Figure 4). This technology surpassed 10,000 hours of salt spray performance on blasted steel and offers overall better performance than traditional ZRPs. The lower zinc levels enhance transfer efficiency up to 80% better than traditional ZRPs and corrosion performance exceeding ISO 12944 and C5 standard.
Conclusion and Recommendations
The proof of concept for lowering zinc levels in zinc-rich primers (ZRPs) has shown promising market success. However, widespread adoption remains a long-term goal. Coatings companies, including PPG, continue to explore ways to reduce zinc content in ZRPs, with further developments expected in the future.
Zinc percentage specifications were introduced decades ago, but given the challenges associated with high-zinc ZRPs and emerging solutions, it is time to revise these standards. Specifications should be based on validated performance data rather than zinc percentage alone. The commercial adoption of lower-zinc primer technologies needs to shift to more performance-based specifications, in contrast to current zinc-level-based specifications. Further discussions are necessary to align regulatory standards, encourage specification modifications and facilitate customer adoption.
Acknowledgements
Susan Donaldson, John Schneider, Nicole Rakers, Travis Bush, Marisol Rodriguez, Denise Callihan and Desiree M Miller.
References
[1] Hussain, A.K.; Seetharamaiah, N.; Pichumani, M.; Chakra, C. S. Research Progress in Organic Zinc Rich Primer Coatings for Cathodic Protection of Metals – a comprehensive review. Progress in Organic Coatings 2021, 153, 106040.
2 Impact.nace.org/economic-impact.aspx
3 Carboline news, BIC Magazine’s June/July 2018 issue: bic-junejuly-2018-hook.pdf
4 Nicole, R; Lim, Mary Lin; McCarthy, Michael; Begley, James; Mubarok, Arif. Efficacy of Zinc Content in Sacrificial Protection Coatings. SSPC Coatings 2021, S2021-00018, 1-10.
5 Tesla Nanocoatings news: Advancements in Zinc Primers for Offshore Protection.
6 Dandan, He; Lida, Wang; Zhengqing,Yang; Wen, Sun; Yixuan, Feng; Kaixin, Xu; Xuesong, Chen; Yine, Ren; Guichang, Liu. A New Understanding of Graphene Influencing the Protective Performance of Zinc-Rich Coatings. Ind. Eng. Chem. Res. 2024, 63, 17, 7661–7672.
7 Niteen, Jadhav; Subramanyam, Kasisomayajula; Victoria, Johnston Gelling. Polypyrrole/Metal Oxides-Based Composites/Nanocomposites for Corrosion Protection. Frontiers in Materials 7, 95.
8 Hempel’s AvantGuard® technology
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!