What is the usual procedure for selecting a defoamer for a new waterborne coating formulation? For many companies, the procedure appears to be as follows: try the defoamers that you currently use; if these don’t work then try samples in the lab, ask a colleague or friend or, maybe, ask a supplier. This approach makes good sense when working with formulations that are similar, as defoamers will usually give consistent performance in similar formulations. However, when these tried and trusted defoamers don’t work, the chemist has the frustrating task of trying to find a suitable product. There is a truism in England that goes, “if all else fails, read the instructions,” however, defoamers don’t usually come with instructions. Wouldn’t it be nice if they did?
Difficulties in finding a suitable defoamer exist because the performance of each defoamer is affected by the formulation it is used in; change the formulation and the defoamer performance may change as well. Defoamer selection is also one of the last steps in readying a formulation for final use, so the rest of the formulation is already mostly decided, and the defoamer has to work within this formulation. This article presents a brief review of defoamer chemistry and formulation, and describes how defoamer performance is affected by other formulation components in the coating. It also introduces a new range of defoamers that provide consistent and predictable performance relative to one other so that the results of an unsuccessful test can be instructional in selecting the next defoamer for testing with a greater chance of success.
Foam Theory
Foam is a dispersion of gas bubbles, usually air, at the surface of a liquid that can be generated in a number of ways, but most commonly by vigorously mixing. A simple shake test with a bottle of water will show that, in pure liquids, these bubbles are not stable and quickly burst, destroying the foam. Small bubbles coalesce into bigger bubbles that rise to the surface, where the bubbles expand due to the greater gas pressure inside the bubbles. This causes each bubble wall or lamella to thin and, ultimately, the bubble will break open as liquid in the lamellae drains under gravity.
However, water-based coating formulations are not pure liquids; they are dispersions of many different materials suspended in water and stabilized by surfactants. These surfactants can also stabilize foam so bubbles can accumulate at the liquid surface as foam (Figure 1).1-3 Once foam is present, it can cause many problems, including reduced production efficiency and higher energy demand, incorrect raw material dosing due to the lower density of foamed material, and incomplete filling of production vessels and product packaging. Foam also affects the application of the coating by reducing the amount of coating applied; bubbles trapped at the surface or inside the dry film will spoil both the surface appearance and protective qualities of the finished coating.
Defoamers are the most widely used method of removing unwanted foam from a coating. Chemical defoamers work by disrupting and breaking the surfactant-stabilized bubble walls to release the trapped air. Most defoamers are complex mixtures of different materials, including:
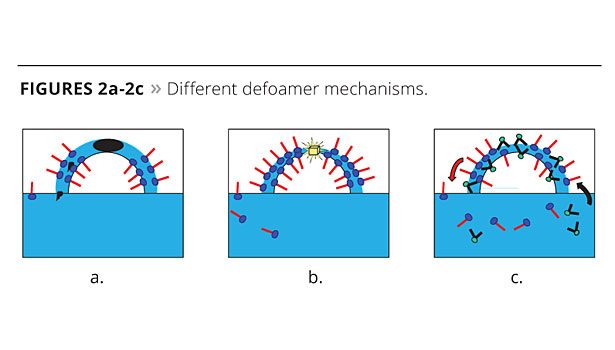
- A carrier fluid that can spread across and bridge the bubble wall, forming an unstable film that is easily ruptured (Figure 2a); the carrier also facilitates the entry of hydrophobic particles into the bubble wall;
- Hydrophobic particles that bridge the lamella and cause rupture by dewetting (Figure 2b);
- Non-foamy surfactants that can displace the foam-stabilizing surfactants at the lamella surface (Figure 2c);
- Other components added to improve defoamer stability, incorporation and compatibility.
These mechanisms have been summarized in detail by Garrett,4 and it is probable that many of these mechanisms are at work when defoaming formulated coatings. Therefore, all the components in a defoamer may be critical to its performance.
Of course, paint formulators are not just concerned with the effectiveness of the defoamer at getting rid of bubbles and foam; the defoamer must eliminate foam without causing undesireable side effects. The same hydrophobic solids and carrier fluids that break the bubbles can also cause problems with the drying paint film, leading to craters and fisheyes. The presence of these components at the surface of a dry (or nearly dry) film can also make it difficult to recoat and creates adhesion problems. It is this balance between effective defoaming and avoiding film defects that can make finding the ideal defoamer for a formulation so challenging and frustrating.
Factors Affecting Defoamer Performance
Figure 3 shows how the performance of five commercial defoamers (represented in different colors) changes in four very different coating formulations: a 35% pigment volume concentration (PVC) alkyd primer formulation from Nuplex; a 55% PVC interior paint formulation from Celanese; a polyurethane (PU)-acrylic hybrid clearcoat for parquet floor lacquer formulation from DSM; and a polyurethane dispersion (PUD) clearcoat for plastic formulation from DSM. The defoaming performance and application quality were measured by different methods for each formulation and application, and then adjusted to a 1-10 scale, where 10 represents perfect performance (no foam and/or perfect film quality) and 1 represents poor performance. The ideal performance is, therefore, shown in the top right hand corner of the graph.
Hegedus reviewed the many different factors that affect the performance of a defoamer in different formulations and highlighted how this information could be used to more effectively guide defoamer selection.5 Higher viscosity and more highly filled (high filler to binder ratio) formulations are harder to defoam but are usually less sensitive to defects. Similarly, fast-drying formulations and coatings applied in thick films are also harder to defoam and often less prone to surface defects, whereas low-viscosity formulations are generally more sensitive to surface defects but easier to defoam. Craters, fisheyes and other defects are also more visible in high-gloss formulations and clearcoats, and often require more careful defoamer selection. Brushes and rollers often create more surface foam when the coating is applied, while spray techniques can often leave bubbles trapped below the film surface (microfoam).6 The substrate is also important; porous substrates such as wood and concrete can be less sensitive to defects but release air into the coating film as the liquid coating wets and penetrates the substrate. Smooth, low-energy surfaces like plastics are harder to wet and more prone to surface defects.
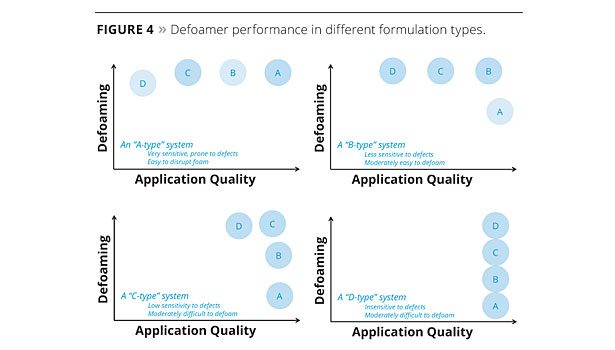
However, even with this understanding, it can still be challenging to find a product that gives acceptable performance with the many defoamer samples that are available. Recently, we have tested many different defoamers in different formulations and observed distinct trends in defoamer performance with different formulation types. These were grouped into four approximate classes (Figure 4) where “D-type” defoamers are the strongest and least compatible defoamers, and “A-type” defoamers are the most compatible products.
New Defoamer Development
Polysiloxane polymers are non-volatile, chemically inert, temperature-stable and highly efficient, and they can control almost all types of foam in any media. Due to the flexibility of the Si-O bonds present in these materials,7 all siloxane backbones offer high spreading coefficients and easy orientation at the interface, while the methyl groups offer both hydrophobicity and low surface tension.8 These factors make siloxane-based defoamers highly efficient because of their low surface tension and fast spreading on the foam system. Polysiloxane polymers can also be chemically modified to improve the compatibility of the defoamer to minimize surface defects and help incorporate the defoamer into the coating formulation. This modification allows a formulator to balance the defoaming power and compatibility of the defoamer within an aqueous system.
A series of eight experimental, polysiloxane-based defoamers (51-58) with the predictable defoaming ability and compatibility balance to fit design targets based on these four different formulation types was developed through understanding these structure-property relationship studies. Defoamers 51 and 52 are the strongest defoamers that fit the D-type profile shown in Figure 4. Similarly, defoamers 53 and 54 are C-type defoamers; defoamers 55 and 56 are B-type defoamers; and defoamers 57 and 58 are the most compatible, A-type, defoamers. We have strategically incorporated a variety of polar organic groups into the polysiloxane backbone and created a series (51-58) of structured siloxane defoamers with a high degree of performance predictability. The goal of this development was to demonstrate that this predictability can be used to aid product selection and improve the efficiency of formulation development by avoiding the trial-and-error approach where data cannot be transferred from each failed experiment.
Figure 5 shows the formulator’s dilemma. The chart on the left shows the defoaming and film quality results from extensive testing of 28 industry benchmark defoamers in a simplified water-based pigment grind (dispersing a phthalocyanine blue 15:3 pigment in a styrene acrylic resin) and mass-tone letdown (1:3 dispersion in acrylic emulsion). All the tested defoamers were recommended in product literature for this type of application. The results are shown adjusted to a simple 1-10 scale, where 1 represents poor film quality or defoaming performance and 10 is excellent performance. The sample containing no defoamer (blank) is shown with a gray circle and had excellent film quality (rated 10) but was extremely foamy (rated 1). The commercial defoamers varied widely in performance with little to no commonality or consistency. There is a roughly equal spread of defoamers that are either too incompatible (good defoaming, poor film quality) or too compatible (good film quality, poor defoaming). Only two of the 28 defoamers tested gave satisfactory overall performance, less than a 10% success rate! This would be a significant time investment and frustration for a formulator trying to choose a suitable defoamer from scratch.
In comparison, the prototype series of defoamers was also tested in the same formulation and standardized to the same scale (chart on the right in Figure 5). While not perfectly linear, a very clear trend is apparent, which tracks very favorably to the predicted defoamer performance of a B-type system shown in Figure 4. One of the products also meets the acceptable performance standards, a success rate of 20%.
In order to meet the goal of reduced experimentation and good predictability of defoamer performance, the series of prototype defoamers was tested in a wide variety of different formulation types to determine if they consistently matched the expected trends shown in Figures 4 and 5. The following examples show how this new series of structured siloxane defoamers matches the theoretical performance variation and provides a significant level of predictability.
The first two examples show the defoamers’ performance in two different interior paint formulations, one flat (70% PVC) and the other semigloss (45% PVC). The defoamers were added during the preparation of the millbase and their performance was measured as a function of grind density, letdown density, as well as any appearance of foam in the final applied film. The film quality and number of surface defects were also measured subjectively. Both defoaming and film quality results were standardized to a 1-10 scale, with the blank (no defoamer) having the worst defoaming results (rated 1) and best film quality (rated 10). The results are shown in Figure 6.
The flat paint formulation is almost completely insensitive to defoamer-related defects, but very difficult to defoam due to the high viscosity and high volume of pigments and fillers relative to the carrier. The results match almost perfectly the D-type system performance outlined in Figure 4 and, as expected, the defoamers with the highest degree of incompatibility, 51, 52 and 53, offer the best performance. The more compatible defoamers are not as effective at defoaming. However, the results in the lower-viscosity, less-filled semigloss paint are quite different because the strongest defoamers created surface defects; the best performance is obtained with defoamers with intermediate incompatibility (B- and C-type) and prototypes 53, 54 and 56, gave the best balance of defoaming efficacy and film quality.
This trend is continued in a clear wood coating formulation based on a PU-acrylic hybrid resin. This system is formulated without pigments, and the defoamers were added to the finished formulation with simple, low-shear mixing. Three coats were applied to sanded red oak panels with a foam brush. The first coat was sanded prior to application of the second and third coats. We would expect this formulation to require a much more compatible defoamer (A-type or B-type), and the results support this prediction exactly, with defoamer 56 giving the best performance. These results are shown in Figure 7. There are no visible defects in the base formulation (no defoamer added) as expected, but the foam trapped in the dried coating is clearly visible. The most compatible A-type defoamer, 58, gives improved foam control and no defects, but there is still room for improvement as foam is still present in the finished film. This defoamer type is not incompatible enough for the demands of this formulation; however, in the lower right picture, the more incompatible C-type defoamer, 54, eliminates foam but also causes numerous craters and surface defects. This defoamer is too incompatible for this formulation. The B-type defoamer, 56 (lower left), has the correct and predictable balance of incompatibility to give optimal defoaming without defects. A quick screening of any one of the three potential defoamer types would allow a formulator to quickly understand the needs of the system and identify which defoamer to try next based on these results, saving time and work.
Formulators of industrial coatings that are applied by continuous methods such as roll coat, curtain coat or even printing methods are also concerned about the persistency of the defoamer during application. Defoamers can often lose performance over time, especially under high shear conditions when the defoamer can become emulsified and broken down into the formulation. In general, stronger defoamers better resist this effect and the high-shear mixing can also help reduce the defects caused by these more incompatible materials.
To demonstrate this, the prototype defoamers were tested in a resinated pigment grind (PR22) and then let down into an acrylic ink formulation. Two other silicone defoamers recommended for ink applications were also tested. The ink was then sheared at 1500 rpm using a Cowles blade over a period of hours and the foam (measured by density) and application quality (measured by drawdown onto Leneta Chart) tested regularly throughout the test. The results are shown in Figure 8. As predicted, the strongest defoamers maintained their defoaming properties longest. The surface appearance of the inks formulated with the stronger defoamers also improved during this time. Initially, defoamers 55, 56 and 57 all gave good appearance, with 55 giving the best balance of defoaming properties and surface appearance. The stronger defoamers all caused craters or dewetting. After 30 minutes shear, the appearance of the ink formulated with defoamer 54 was also acceptable, and this remained the best performer over time.
Conclusion
A series of modified siloxane defoamers has been developed that offers a controlled range of defoaming strength and compatibility relative to each other. By understanding the basic formulation demands such as shear, PVC level, sensitivity and viscosity, a formulator can quickly select one product from the series to test first and then use the results of this test as instructions to direct the selection of the next defoamer to test, should the first product not give acceptable performance. If the first product shows evidence of defects, then a more compatible defoamer is required. Conversely, if the first product shows inadequate defoaming, then a product in the series that is more incompatible is desired.
References
- Jönsson, B.; Lindman, B.; Holmberg, K.; Kronberg, B. “Foaming of Surfactant Solutions”, In Surfactants and Polymers in Aqueous Solution, Eds. John Wiley & Sons, New York, 1998, 325-336.
- Wasan, D.T.; Koczo, K.; Nikolov, A.D. “Mechanisms of Aqueous Foam Stability and Antifoaming Action with and without Oil,” In Foams: Fundamentals and Applications in the Petroleum Industry, Schramm L L, Ed., American Chemical Society, Washington, D.C., 1994, Advances in Chemistry Series 242, 47-114.
- Rosen, M. J. Surfactants and Interfacial Phenomena, 2nd Ed., John Wiley & Sons, New York, 1989.
- Garrett, P.R. “The Mode of Action of Antifoams,” In: Defoaming: Theory and Industrial Applications, Garrett P R, Ed., Taylor & Francis Group, LLC, Boca Raton, FL, 1992, Surfactant Science Series 45, 1-117.
- Hegedus, C. R.; Reader, J.; Lai, K.T.G. Optimal Defoamer Selection for Coatings: Guidelines and Case Studies, Proceedings of ABRAFATI 2011, São Paulo, Brazil, November 21-23, 2011.
- Louis, C.; Reinartz, R.; Chaigneau, W.; Reader, J.; Lai, G. New De-aerators for Airless Spray Applied Waterbased Coatings, Proceedings of the European Coatings Congress, Nürnberg, Germany, March 30, 2011.
- Grigoras, S. In: Computational Modelling of Polymers, Bicerano J, Ed., Marcel Dekker, New York, 1993, 161.
- Voronkov, M.G.; Mileshkevich,V.P.; Yuzhelevskii, Y.A. The Siloxane Bond, Consultants Bureau, New York, 1978.










Report Abusive Comment