In the 1950s, Dr. Herbert Koch from the Max Plank Institute in Mülheim, Germany, found that olefins may react with carbon monoxide and water under the influence of strong acids to form tertiary branched neocarboxylic acids (Figure 1). Before the intermediate carbocation reacts with carbon monoxide, isomerization reactions are observed and, therefore, the resulting acid is composed of a number of isomers.1,2
The neocarboxylic acid can be converted into its vinyl ester monomer by reaction with acetylene. Today, these monomers are marketed under the trade name VeoVa™ vinyl ester and are also widely referred to in industry as vinyl versatate monomers, neo-esters or VV monomers.
VeoVa vinyl ester monomers are very hydrophobic vinyl esters with a highly branched tertiary substituted α-carbon structure. Their principal use is as hydrophobic co-monomers in vinyl and acrylic polymerization. Their alkyl neocarboxylic group is very resistant to degradation in alkaline conditions, as there is no hydrogen on the α-carbon atom. The branched tertiary structure with bulky and hydrophobic hydrocarbon groups provides the neo-ester monomers (Figure 2) with a highly hydrophobic nature and a low surface tension. Furthermore, they possess a strong resistance to hydrolysis and do not degrade under the influence of UV light.
The VeoVa vinyl ester monomers easily polymerize with various other co-monomers through reaction of the vinyl ester functional group. In this way the specific properties of the monomer can be imparted to its copolymers. VeoVa monomer enhances the performance of vinyl acetate- and acrylic-based latices, significantly upgrading key properties such as water and alkali resistance in both polymer systems.
VeoVa vinyl ester-based polymers exhibit the required polymer hardness and flexibility balance, hydrophobicity and chemical resistance for the formulation of a wide range of latex coatings. The resulting paints are characterized by very good water, UV and alkali resistance, and therefore exhibit very good outdoor durability.3 VeoVa monomers already have been successfully used to manufacture VeoVa/vinyl acetate copolymer latices. Used as binders for architectural paints, these latices provide improved scrub resistance and exterior durability. These properties of hydrophobicity, resistance to hydrolysis and UV light make VeoVa monomers also particularly suitable for producing high-performance latices, especially when they are copolymerized with acrylic and methacrylic monomers. VeoVa vinyl ester-modified acrylics can be formulated in protective coatings such as anti-corrosion paints, water-repellent systems, wood coatings, elastomeric roof coatings and adhesive applications such as PSAs.
Glass Transition Temperature
The various VeoVa monomers mainly differ in the degree of branching and the length of the hydrocarbon groups. This leads to differences in the glass transition temperature (Tg) of their homopolymers and consequently to different copolymer properties. The broad range of Tgs available within the portfolio of VeoVa monomers offers an important tool to the polymer chemist to develop hydrophobic polymers within a very wide Tg range.
VeoVa 9 monomer is the vinyl ester of neononanoic acid (9 carbon atoms) and is considered a harder monomer as it imparts a Tg of +70 °C. Scholten and Van Westrenen illustrated the effect of chain branching by measuring the Tg of a series of polymer prepared from VeoVa 9 vinyl ester isomers.4 The Tgs were found to range from +10 to 119 °C, leading to the conclusion that the high Tg of poly-VeoVa 9 is a cumulative effect of the shorter chain length and the higher degree of branching within the various isomer mixtures.
VeoVa 10 monomer is the vinyl ester of neodecanoic acid (10 carbon atoms) and has a homopolymer Tg of -3 °C, making it a flexibilizing monomer. VeoVa EH monomer is the vinyl ester of 2-ethylhexanoic acid, a more linear acid, and therefore has a significantly lower Tg of -36 °C
Water Resistance
Water resistance is one of the most important barrier properties of a coating. The water resistance of a coating is mainly governed by its polymeric binder and the monomers used to produce the binder. If water solubility of the monomers is taken as an indication of hydrophobicity (Table 1) it becomes clear that VeoVa monomers have a much higher hydrophobicity than other monomers that are commonly used in emulsion polymerization.5
Ease of Polymerization
VeoVa monomers can be used in combination with a variety of other monomer types to produce various polymers. The ease of polymerization depends on the reactivity ratios of the monomers used (Table 2).
The data reported in Table 2 show that VeoVa monomers have a similar reactivity to the vinyl acetate monomer. All the vinyl esters have a similar reactivity independent of the size of the carboxylic group. There is also a difference of reactivity between the vinyl ester monomers and (meth)acrylate monomers, but this difference is such that they can still react together in emulsion polymerization. Finally, the difference of reactivity between the vinyl ester monomers and styrene is so large that they cannot copolymerize in emulsion polymerization.
In summary, the VeoVa monomers copolymerize easily with other vinyl esters, ethylene and most acetate-based and acrylic-based monomers.
VeoVa-Modified Vinyl Acetate-Based Latices
VV10 and vinyl acetate are very much complementary to each other with respect to the properties they impart to latex polymers. Vinyl acetate homopolymers, although economic in use, lack the flexibility and durability required for most emulsion paint applications. Homopolymers of VV10 (Table 3), on the other hand, exhibit very good resistance to hydrolysis, UV light and water, but having a Tg of -3 °C they are too soft for most coating applications. Copolymers of vinyl acetate and VV10 provide a well-balanced set of performance characteristics.
Polyvinyl acetate has a Tg of +38 °C and is therefore brittle at ambient temperature. Co-polymerization of VV10 permanently flexibilizes the polymer and reduces the minimum film formation temperature (MFFT).
Protective Effect of the VeoVa Vinyl Ester Molecule
VV10 and vinyl acetate exhibit a very similar reactivity (r1=0.99; r2=0.92) in radical polymerization reactions, which leads to polymers with the monomer units randomly distributed along the chain. This is important, because it allows the key performance characteristics of the VV10 monomer to be fully exploited. The unique, highly branched, carbon-rich structure of the monomer sterically protects its ester group from being hydrolyzed. More importantly, it also protects neighboring acetate groups (Figure 3), thus improving the hydrolytic stability of the polymer. This protection phenomenon is referred to as the “umbrella effect”. This enables such polymers to be successfully used as paint binders on even very alkaline substrates.
The umbrella protection effect of the highly branched carboxylate groups is supported by experimental data. The alkali resistance of a latex can be determined by the percentage of ester groups hydrolyzed after four days immersion in a 2% aqueous solution of sodium hydroxide.
The results (Figure 4) show clearly that the introduction of VV10 in vinyl acetate-based polymers significantly improves the alkali resistance of both colloid-stabilized and colloid-free systems. While only 20% of VV10 already has a profound positive influence, a 30% use is recommended to obtain the very good alkali resistance required for high durability of emulsion paint on alkaline surfaces.
Vinyl acetate homopolymer, as well as its copolymers with butyl acrylate or 2-ethylhexyl acrylate, tend to hydrolyze easily and almost completely with time. The data shows that the alkali resistance of a VA/VV10 (85/15) is significantly better compared to the alkali resistance of a VA/2-EHA (85/15), and the same is observed for a VA/VV10 (75/25) compared to a VA/BA (75/25). VV10 is therefore far more effective in protecting the acetate group than an acrylate monomer such as 2-ethylhexyl acrylate or butyl acrylate. This can be explained by the fact that the neo-acid structure is more bulky, apolar and more effectively randomly located onto the polymer backbone. Equally, the alkali resistance of a vinyl acrylic latex can also be improved by the copolymerization of VV10. As can be seen from Figure 5, dry VV10-based latex films immersed for two weeks in a 2% NaOH solution remain almost unchanged. The alkali extractables from a latex clear film of the VeoVa-based terpolymer can be as low as the one of an all-acrylic or styrene-acrylic, and definitively better than a vinyl acrylic.
Effect on Wet Scrub Resistance
Vinyl acetate-rich polymers tend to soften and weaken considerably under the influence of water because vinyl acetate has a very hydrophilic characteristic. Hence, such systems may fail during wet cleaning or scrubbing. This is much less the case when the polymer contains a sufficient amount of hydrophobic monomer. VV10 performs better in this respect than other co-monomers for vinyl acetate. A series of vinyl acetate/VV10 binders with different VV10 content was formulated in a 60% PVC matte paint. The scrub resistance improved with increasing VV 10 content of the binder, as shown in Figure 6.
Outdoor Durability
Exterior paints first and foremost need to be resistant to the influence of the weather, such as fluctuations in temperature and humidity and to the effects of UV light. Equally important is that the paints resist exposure to alkali (e.g., from the substrate) and accommodate dimensional changes. VV10 latices, when properly formulated, have proven to be among the best performing under severe conditions. VV10, unlike other co-monomers for vinyl acetate, provides the hydrophobicity and hydrolytic stability required to resist degradation of the binder from exposure to alkaline substrates. Both vinyl acetate and VV10 impart good UV resistance. As can be seen in Figure 7, the VV10-based binders have much better outdoor durability than other vinyl acetate-based binders. Moreover, a VA/VV10 (70/30 m/m)-based paint equals or even outperforms the more expensive acrylic-based paints in erosion resistance and yellowing resistance. Also a vinyl acetate/VV10/BA (74/28/6) terpolymer-based paint performed very well in this 10-year exposure test. The paint with the styrene acrylic binder remained intact but severe yellowing was observed.
VeoVa Vinyl Ester-Modified Acrylics
VeoVa monomers readily copolymerize in emulsions with acrylates and methacrylates. The bulky and hydrophobic hydrocarbon structure of the VeoVa monomers enhances the polymer to provide a high degree of water repellence and hydrolysis resistance. The small particle size of the latices (~100 nm) facilitates film formation. VeoVa/acrylic latices represent a large family of polymers with a wide range of possible polymer compositions and performance properties.
Water Resistance of Acrylic Polymers
Because of their hydrophobic nature, both VV10 and VV9 can increase the water resistance of their copolymers. The amount of water absorbed and also the whitening of the polymer film after exposure to a drop of water can be used as a measure for the water repellence of a coating. However, the polymer Tg and the surfactants used can influence these tests. To demonstrate only the effect of the VeoVa monomers we tested a series of polymers having the same Tg and surfactant system. The Tg was kept constant by adjusting the concentration of either the MMA or the BA monomers. Figure 8 shows the influence of the VeoVa monomers on water absorption after 14 days immersion.
Whitening of the film was measured by the water spot test. A drop of water is applied on the clear film and the whitening of the water droplet is rated visually after one day. Figure 9 shows the influence of the VeoVa monomers on water spot resistance after 24 h of exposure.
It can be seen from Figure 8 and Figure 9 that any incorporation of VeoVa monomers leads to significant improvements in water resistance and the effect increases with increasing VeoVa content. For both water absorption and water spot resistance, the best results are obtained by using VV9, a harder monomer than VV10. At the same VeoVa concentration, more MMA is needed with the VV10-based systems to obtain the same Tg as with the VV9 polymer. Since MMA is a more polar or hydrophilic monomer than BA, an increased MMA content will increase water sensitivity of the terpolymer. It can be seen from Figure 8 and Figure 9 that any incorporation of VeoVa monomers leads to significant improvements in water resistance, and the effect increases with increasing VeoVa content.
The water repellence effect can also be demonstrated visually in a water beading test, measuring contact angles. Figure 10 shows the spreading of equally sized droplets of water over various acrylic paints. Acrylic systems modified with 30% VeoVa had a high water contact angle of 79°. On the VeoVa-modified acrylic paint the water beads to a small droplet, while on pure acrylic systems containing either butyl acrylate or 2-ethylhexyl acrylate the water readily spreads out over a large area of the paint surface.
Expensive additives such as special waxes or silicones are commonly formulated into coatings to provide them a water beading effect. Often, the effect of these additives is only temporary, as they will gradually leach out of the coating. When the hydrophobic component is bound chemically to the polymer backbone (e.g. using VeoVa), the efficacy is extended.
Water Vapor Transmission
Water vapor permeability is another important property in coatings and adhesives. For optimum protection a low transmission of water vapor is required. Water vapor transmission of VeoVa/acrylic polymers was determined according to ASTM method D1653, with the dry cup method. The polymer consisted of VAM/2-EHA/VeoVa polymers, and the effect of VeoVa was determined by replacing equal amounts of the already relatively hydrophobic 2-EHA with VV10. It can be seen in Figure 11 that there is an almost linear correlation with the VeoVa content and the water vapor transmission rate. The more 2-EHA that is replaced by VeoVa the less water migrates through the polymer.
Applications
Virtually all coating applications will benefit from increased water resistance. Very good performance for VV/acrylic polymers has been demonstrated in metal protection paints, wood coatings, concrete tile coatings, exterior architectural paints and water-repellent systems, as well as in adhesives such as pressure sensitive adhesives (PSAs). In addition the global drive to decrease VOC levels requires the need for polymers with a low Tg and reduced MFFT. As lower-Tg polymers usually suffer from higher water sensitivity, VeoVa vinyl ester provides a tool to keep excellent water resistance in low-VOC systems.
Metal Protection
The good adhesion to metal and high hydrophobicity makes VeoVa monomers very suitable for waterborne and VOC-compliant industrial applications such as metal protection.6 An emulsion polymer was prepared, having a monomer composition of VV10/MMA/BA/AA 60/35/2/3 with a MFFT of 32 °C to investigate the performance of VeoVa/acrylic binders in anti-corrosion paints. As a reference we evaluated a commercial styrene/acrylic latex (MFFT 30 °C) advertised for use in anti-corrosion applications. The emulsions were formulated in a 23% PVC coating that can be used as a primer or eggshell topcoat. The primers were applied at 100 micron dry film thickness on cold rolled steel (Q panels R46). Salt spray testing was performed according to the ASTM B 117-90 method. After 750 h, the test was stopped and pictures of the panels were taken (Figure 12). One can see that blistering only occurred around the scribe for the VeoVa-based primer. On the other hand, the styrene/acrylic-based primer shows very severe blistering over the entire surface of the panel.
Hydrophobic Pressure Sensitive Adhesives
Acrylic emulsion polymers were modified by varying the level (0, 10 and 20 wt%) of VV10. A series of these three latices with a targeted Tg (~ -40 °C) were prepared under the same polymerization conditions keeping the surfactants, functional monomers types and levels constant. These were then characterized and tested as a PSA.
The products based on the hydrophobic vinyl ester are less affected in a humid environment as can be observed from the peel test performed on the PSA samples applied for seven days under 90% relative humidity and 35 °C (Figure 13) vs. samples stored under standard conditions.
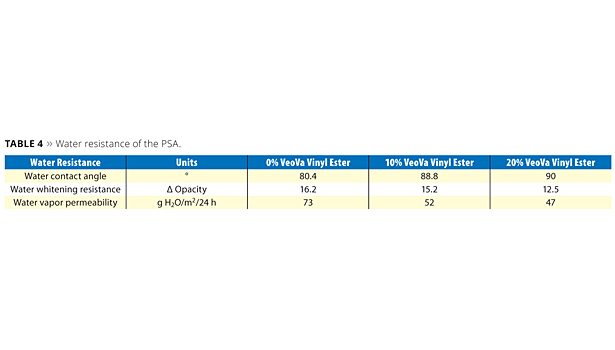
On this basis, further investigation of the hydrophobic nature of the different latexes was pursued. A few specific evaluations related to the water resistance were performed (Table 4).
The water contact angle measurement is an easy way to assess the polar character of the PSA film surface. From the data obtained, it appears that the modification of the acrylic backbone with the hydrophobic vinyl ester considerably decreases the polarity of the PSA film surface.
It is important that the PSA for filmic labels show good resistance against water whitening when exposed to moisture. Results obtained are shown in Figure 14, where pictures of the PSA films directly exposed to water are given after 24 h of contact. The pictures clearly demonstrate the reduced water whitening provided by the sample containing 20% of VV10. The opacity delta of the different samples was measured with a colorimeter in order to quantify the degree of whitening resistance (Table 4). Typically a lower delta of opacity value indicates better resistance against water whitening. The improvement brought by the hydrophobic vinyl ester can be determined and confirms the qualitative visual evaluation.
The water vapor permeability of the different PSAs was investigated to further assess the efficiency of the modified polymer to act as a barrier against water vapor. Here again, it can be observed that the hydrophobization of the polymer has a noticeable effect on the diffusion of water through the PSA.
Elastomeric Roof Coatings
Elastomeric roof coatings have been used for many years to extend the useful life of many types of roofing substrates. The roof coatings market also has seen significant growth, driven by the cool roof movement. Thermoplastic polyolefin (TPO) roofing membranes are a popular roofing system in the United States. It has been a challenge developing a waterborne elastomeric roof coating formulation with sufficient adhesion to the polyolefin surface of weathered TPO roofing membranes. Since VeoVa monomers lower the surface tension and increase the hydrophobicity of the polymers they are incorporated to, VeoVa-modified acrylics make good candidates for water-resistant elastomeric roof coatings with a good adhesion to polyolefinic substrates.
A series of VV10-modified (0, 15 and 30 wt%) acrylic polymers at an iso Tg of ~ -35 °C were formulated in an elastomeric roof coating and applied on UltraPly™ TPO from Firestone building products. Both wet and dry adhesion was tested (ASTM C794) at a peel rate of 2 in/min. Wet adhesion was measure after the samples were being immersed in water for 7 days.
As can be seen from Figure 15, the incorporation of VV10 has a positive effect on both the dry and wet adhesion of the roof coating to TPO, with higher levels of VV10 leading to further improvements in adhesion. The low surface tension of the VeoVa-based polymers leads to an improved wetting of the TPO surface while the increased hydrophobicity allows maintaining good adhesion under wet conditions.
Exterior Wood Coatings
Without some type of protective surface coating, most wood will deteriorate very rapidly during outdoor exposure. In addition to excellent resistance to weathering and UV, other features such as good adhesion to wood, protection against liquid water ingress and a good balance between hardness and flexibility are key requirements for exterior wood coatings. Moisture protection, however, remains a critical parameter still to be optimized in the field of waterborne acrylic wood coatings.
A series of five self-crosslinkable core/shell acrylic latices with a fixed core Tg of 54 °C and shell Tg of 0 °C (Binders 1-5) were prepared, characterized and formulated into wood stains. For all the systems, the weight ratio of hard to soft material was fixed at 30/70. The monomer composition of the shell is composed of butyl acrylate, methyl methacrylate, acrylic acid, a wet adhesion promoter monomer and either VV10 or 2-ethylhexyl acrylate as hydrophobic monomers at a level of 30 wt% on total monomers
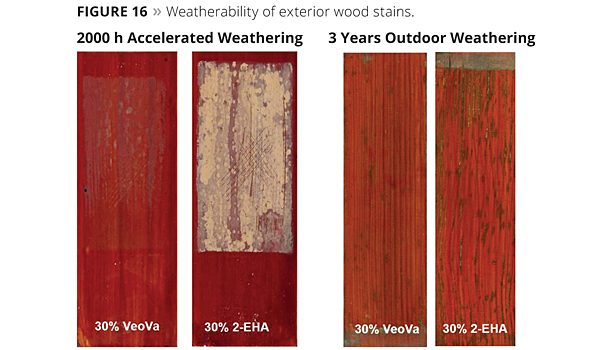
The weathering performance of the wood stains was evaluated with an accelerated weathering test according to EN 927-6. The performance of the wood stains was also assessed by performing an outdoor durability test. This entailed exposure to continental European weather conditions in Belgium for a period of three years (Figure 16).
After 2000 h, no blistering, cracking, flaking or chalking could be observed for wood stain based on the VeoVa-modified polymer. Severe chalking was observed for the wood stain containing 30% of 2-EHA as hydrophobic monomer. Also after three years of outdoor exposure the wood stain based on the VeoVa-modified acrylic performed better than the pure acrylic polymer containing 2-EHA. The superior combination of UV stability with increased hydrophobicity conferred by the VeoVa vinyl ester incorporated into the polymer backbone contributes to the improved weathering resistance of the coating appearance.7
Conclusion
The copolymerization of hydrophobic vinyl ester of branched neo-carboxylic acids significantly improves the performance of vinyl and acrylic binders. Manufacturing of vinyl acetate/VeoVa vinyl ester polymers is easy due to the favorable reactivity characteristics of the VeoVa monomer and vinyl acetate. Incorporating the highly branched structure enhances the alkaline and water resistance of the vinyl copolymers. The resulting paints will offer a high scrub resistance combined with excellent weathering resistance. Combining VeoVa monomers with acrylate monomers offers an additional tool to formulate a diversity of very versatile polymers for use in coating and adhesive applications. The upgraded acrylics perform very well on even the most demanding substrates such as metal, wood and even plastics.
References
1 Koch, H. Production of carboxylic acids from olefins, US patent 2,831,877, filed 17 March 1952.
2 Koch, H.; Fette, Seifen, Anstrichmittel. Über neuere bei der Synthese verzweigter Carbonsäuren erzielte Ergebnisse,Vol 59, issue 7, p. 493-498, 1957.
3 Vandenzande, G.; Smith, O.; Basett, D. Vinyl acetate polymerization in Emulsion polymerization and emulsion polymers, Peter A Lovell, Wiley, 1997.
4 Scholten;Van Westrenen, W.J. Vinyl and Glycidyl Esters in Modern Binder Design, Paint Ink Int., Nov.,1991, p. 8.
5 Basett, D. Hydrophobic coatings from emulsion polymers, Journal of Coatings Technology, January 2001, p. 43-55.
6 Decocq, F.; Slinckx, M.; Nootens, C. A new technology for environmentally friendly and UV-resistant waterborne anti-corrosion paints, Journal of Protective Coatings & Linings, June 2001, p. 48-56.
7 Havaux, N.; Vanaken, D.; Simal, F. Paint & Coatings Industry, October 2010.
For more information, e-mail Victor.arriaga@momentive.com or David.vanaken@momentive.com.






















Report Abusive Comment