Modification of Acrylic Emulsion Polymers with Vinyl Neo-Esters
Enhancing Performance and Providing a Route to Cost-Efficient Alternatives

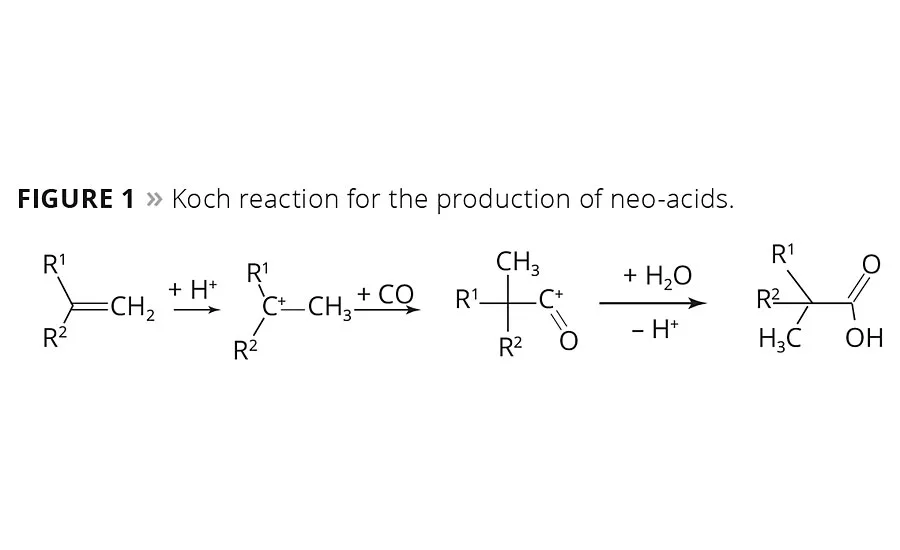
Figure 1
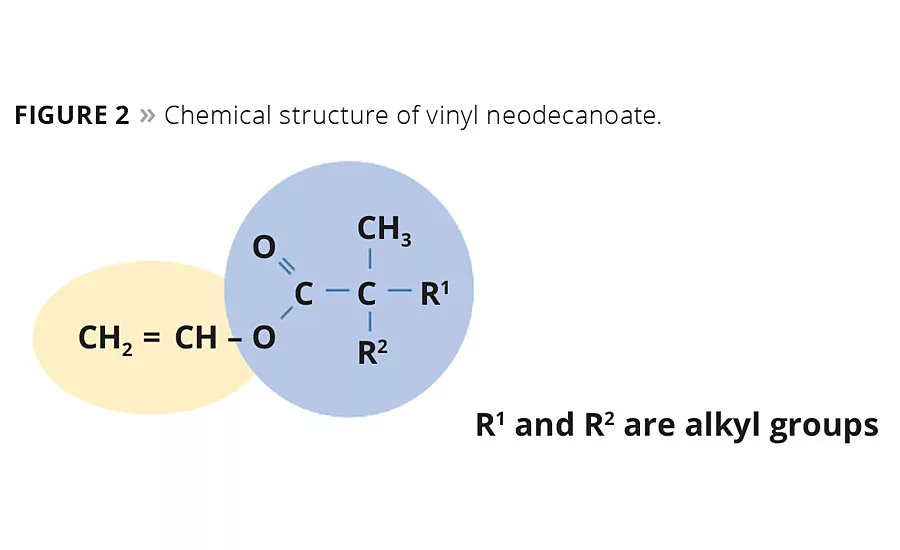
Figure 2
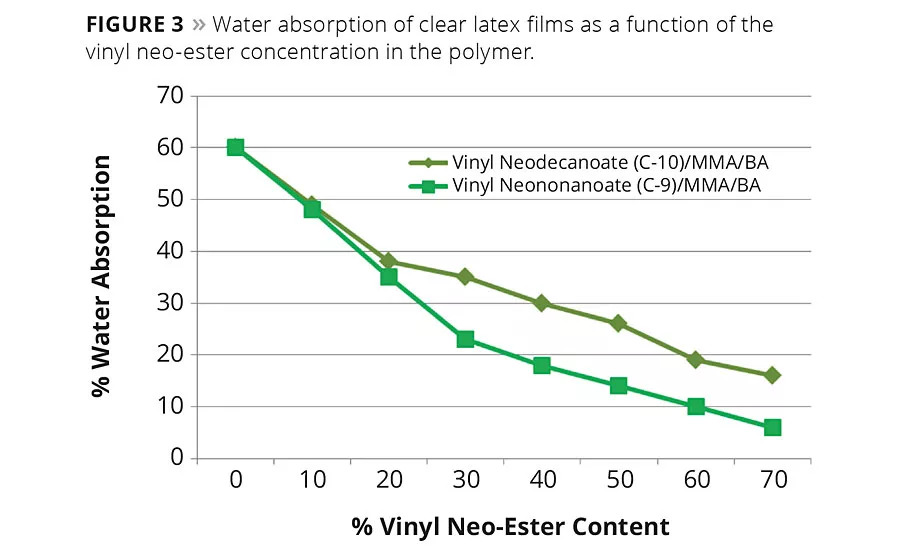
Figure 3

Figure 4
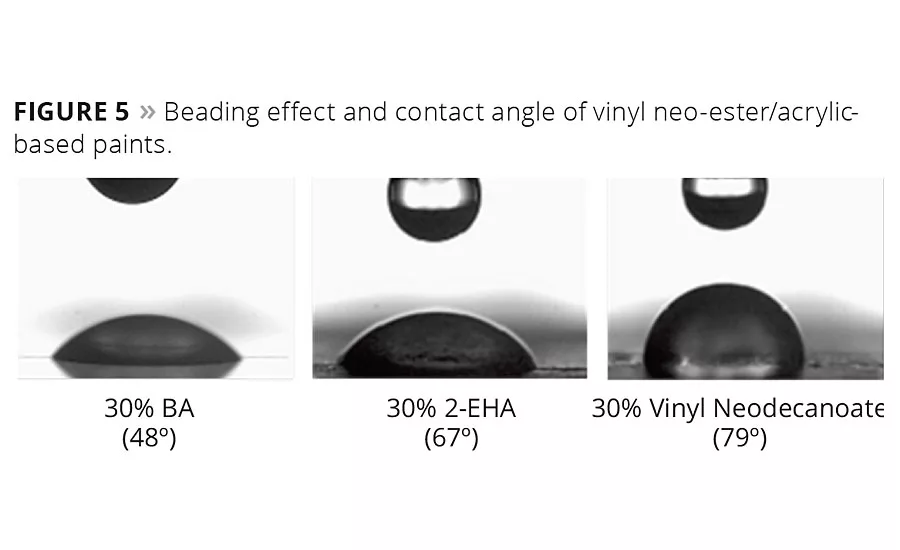
Figure 5
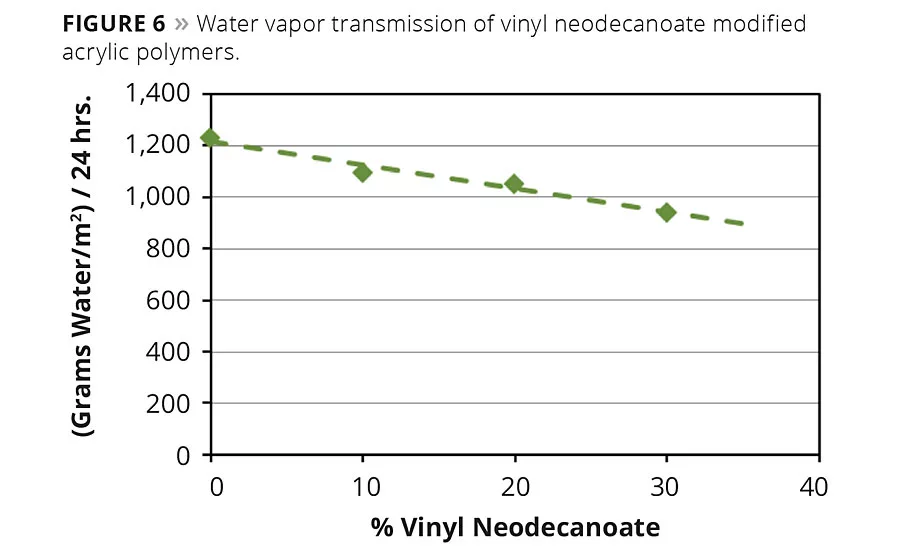
Figure 6
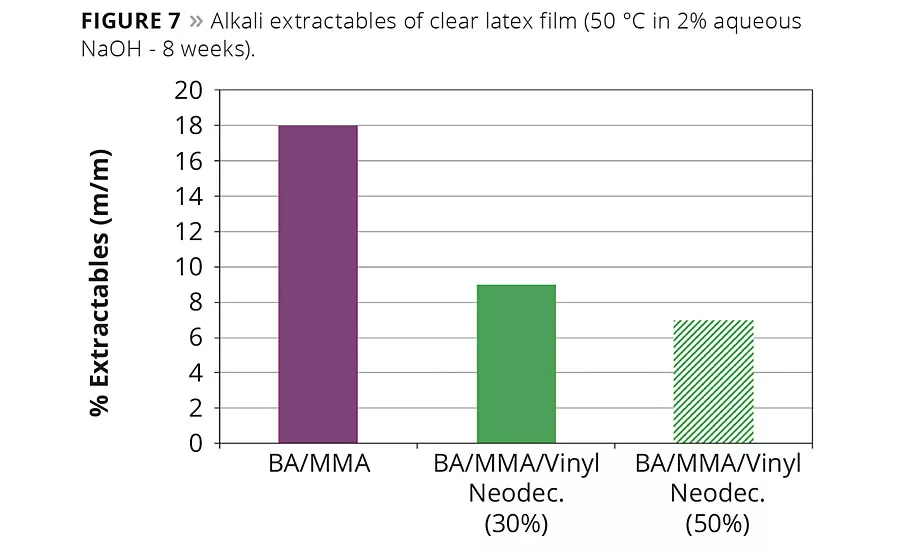
Figure 7

Figure 8

Figure 9

Figure 10
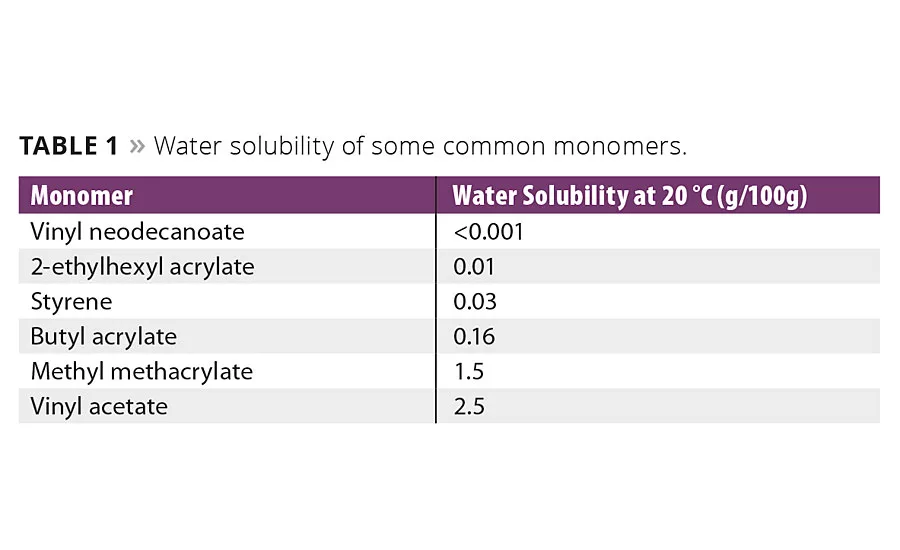
Table 1
In the 1950s, Dr. Herbert Koch from the Max Plank Institute in Mülheim, Germany, found that olefins may react with carbon monoxide and water under the influence of strong acids to form tertiary branched neocarboxylic acids (Figure 1). Before the intermediate carbocation reacts with carbon monoxide, isomerization reactions are observed and, therefore, the resulting acid is composed of a number of isomers.1,2
The neocarboxylic acid can be converted into its vinyl ester monomer by reaction with acetylene. Vinyl neo-esters are very hydrophobic monomers with a highly branched tertiary substituted a-carbon structure. Their principal use is as hydrophobic co-monomers in vinyl and acrylic polymerization. The alkyl neocarboxylic group of these monomers is very resistant to degradation in alkaline conditions, as there is no hydrogen on the a-carbon atom. The branched tertiary structure with bulky and hydrophobic hydrocarbon groups provides neo-ester monomers (Figure 2) with a highly hydrophobic nature and low surface tension. Furthermore, these vinyl neo-esters exhibit strong resistance to hydrolysis and do not degrade under the influence of UV light.
Vinyl neo-esters easily copolymerize with various other monomers through the vinyl ester group. In this way, the specific properties of the monomer can be imparted to its copolymers. Vinyl neo-esters enhance the performance of vinyl acetate- and acrylic-based latices, significantly upgrading key properties such as water and alkali resistance in both types of polymer systems.
Polymers based on vinyl neo-esters exhibit the required balance of polymer hardness and flexibility, hydrophobicity and chemical resistance for the formulation of a wide range of latex coatings. The resulting paints are characterized by very good water, UV and alkali resistance and therefore exhibit very good outdoor durability.3 Vinyl neo-esters have already been successfully used to manufacture vinyl acetate copolymer latices. Used as binders for architectural paints, these vinyl copolymer latices provide improved scrub resistance and exterior durability.
The hydrophobicity and resistance to hydrolysis and UV degradation imparted by vinyl neo-ester monomers make them particularly suitable for producing high-performance latices, especially when copolymerized with acrylic and methacrylic monomers. Acrylic emulsions modified with vinyl neo-ester monomers can be formulated into protective coatings such as anticorrosion paints, water-repellent systems, wood coatings, elastomeric roof coatings and adhesive applications such as pressure sensitive adhesives (PSAs).
Glass Transition Temperature
The two most common vinyl neo-ester monomers are the vinyl ester of neodecanoic acid, which contains 10 carbon atoms, and the vinyl ester of neononanoic acid, which contains nine carbon atoms. The vinyl ester of neodecanoic acid has a homopolymer Tg of -3 °C, making it a flexibilizing monomer, while the vinyl ester of neononanoic acid has a homopolymer Tg of 70 °C. The reason behind the striking difference in glass transition temperatures between these monomers was elucidated by the work of Scholten and Van Westrenen.4 This work evaluated the effect of chain branching by measuring the homopolymer Tg of a series of vinyl esters based on neononanoic acids with differing degrees of branching. The result was a series of polymers with Tgs ranging from 10 °C to 119 °C, leading to the conclusion that the higher Tg of polyvinyl neononanoate is a cumulative effect of the shorter chain length and the higher degree of branching within the various isomer mixtures. This large disparity in glass transition temperature between these vinyl neo-ester monomers allows the polymer formulator the latitude to develop very hydrophobic polymers with a broad latitude of possible Tgs.
Water Resistance
Water resistance is one of the most important barrier properties of a coating, and is mainly governed by the polymeric binder and by the nature of the monomers used to produce the binder. If one takes water solubility as an indication of hydrophobicity (Table 1) it becomes clear that the vinyl esters of neo-acids are much more hydrophobic than other monomers commonly used in emulsion polymerization.5
Acrylic Resins Modified With Vinyl Neo-Esters
Vinyl neo-esters readily copolymerize in emulsions with acrylates and methacrylates. The bulky and hydrophobic hydrocarbon structure of vinyl neo-ester monomers enhances polymers to provide a high degree of water repellence and hydrolysis resistance. Vinyl neo-ester monomer-modified acrylic latices represent a large family of polymers with a wide range of possible polymer compositions and performance properties.
Water Resistance of Acrylic Polymers
Because of their hydrophobic nature, vinyl neodecanoate and vinyl neononanoate can increase the water resistance of the copolymers in which they are used. The amount of water absorbed and also the whitening of the polymer film after exposure to a drop of water can be used as a means to measure the water repellence of a coating. However, polymer Tg and the surfactants used can influence these tests. To demonstrate the effect of the vinyl neodecanoate and vinyl neononanoate monomers independently of other variables, a series of polymers having the same Tg and surfactant system were tested. The Tg was kept constant at 20 °C by adjusting the concentration of the methyl methacrylate (MMA) and butyl acrylate (BA) constituents of the polymers. Figure 3 shows the influence of vinyl neodecanoate and vinyl neononanoate monomers on water absorption after 14 days immersion.
Whitening of the film was measured by the water spot test. A drop of water is applied on the clear film, and the whitening of the water drop is rated visually after one day. Figure 4 shows the influence of the vinyl neo-ester monomers on water spot resistance after 24 hrs of exposure.
Figures 3 and 4 show that the incorporation of vinyl neo-ester monomers significantly improves water resistance and that the effect increases with the neo-ester monomer concentration. The best results in water absorption and water spot resistance are obtained by using the harder monomer, vinyl neononanoate. At the same vinyl neo-ester concentration, more MMA is needed with the vinyl neodecanoate monomer-based system to obtain the same Tg as a the harder vinyl neononanoate system. Since MMA is a more polar or hydrophilic monomer than BA, an increased MMA content will increase water sensitivity of the terpolymer.
The water repellence effect can also be demonstrated visually by measuring water droplet contact angles. Figure 5 shows the spreading of equally sized droplets of water on various acrylic paints. Acrylic systems modified with 30% vinyl neo-ester monomer had a high water contact angle of 79°, and the water beads to a small droplet. With the pure acrylic systems containing either butyl acrylate or 2-ethyl hexyl acrylate, the water readily spreads out over a large area of the paint surface.
Expensive additives such as special waxes or silicones are commonly formulated into coatings to provide them with a water beading effect. Often, the effect of these additives is only temporary, as they will gradually leach out of the coating. When the hydrophobic component is bound chemically to the polymer backbone, such as is the case for vinyl neo-ester monomer-containing polymers, the efficacy is extended.
Water Vapor Transmission
Water vapor permeability is another important property of coatings and adhesives. For optimum substrate protection, low transmission of water vapor is required. Water vapor transmission rate for acrylic polymers containing vinyl neo-ester monomers was determined according to ASTM method D1653, with the dry cup method. The polymer consisted of methyl methacrylate, 2-ethylhexyl acrylate (2-EHA) and vinyl neodecanoate monomer, and the effect of the vinyl neo-ester monomer was determined by replacing equal amounts of the already relatively hydrophobic 2-EHA with vinyl neodecanoate. Figure 6 shows that there is an almost linear correlation between the vinyl neodecanoate content and the water vapor transmission rate. The more 2-EHA that is replaced by vinyl neodecanoate, the less water migrates through the polymer.
Alkali Resistance
Good alkali resistance is required for coatings designed for application on alkaline substrates, such as cement or plaster. Due to the bulky structure of vinyl neo-ester monomers, the ester bond is sterically shielded from alkali-induced hydrolysis and, furthermore, the neodecanoate structure also protects neighboring monomer units within a polymer. To demonstrate this effect, butyl acrylate-methyl methacrylate copolymers were modified with two different levels of vinyl neodecanoate, and clear latex films were cast and subsequently immersed in 2% aqueous sodium hydroxide for 8 weeks at 50 °C. After immersion, the clear polymer films were rinsed, dried and weighed to determine the amount of polymer degraded by hydrolysis. As can be seen in Figure 7, the polymers containing vinyl neodecanoate demonstrate superior resistance to alkaline hydrolysis when compared to the unmodified methyl methacrylate-butyl acrylate copolymer.
Applications
Virtually all coating applications will benefit from increased water resistance. The excellent performance of vinyl neo-ester-modified acrylic polymers has been demonstrated in metal protection paints, wood coatings, concrete tile coatings, exterior architectural paints and water-repellent systems, and also in adhesives such as PSAs. In addition, the global drive to decrease VOC levels requires polymers with low Tg and reduced minimum film-forming temperature (MFFT). As polymers with lower Tg usually suffer from higher water sensitivity, vinyl neo-esters provide a tool to provide excellent water resistance in low-VOC systems.
Metal Protection
The excellent adhesion to metal and high hydrophobicity imparted by vinyl neo-esters makes them very suitable for waterborne and VOC-compliant industrial applications such as metal protection.6 To investigate the performance of vinyl neo-ester-modified acrylic binders in anticorrosion paints, an emulsion polymer was prepared with a monomer composition of vinyl neodecanoate/MMA/BA/AA in the following ratios, 60:35:2:3. This emulsion had a measured MFFT of 32 °C. As a reference, a commercial styrene/acrylic latex (MFFT 30 °C), advertised for use in anticorrosion applications, was also evaluated. Both emulsions were formulated in 23% PVC paints that can be used as a primer or eggshell topcoat. The paints were applied at 100 microns dry film thickness on cold rolled steel (Q-panel R46). Salt spray testing was performed according to the ASTM B 117‑90 method. After 750 hrs, the test was stopped and pictures of the panels were taken (Figure 8). One can see that the primer based on the vinyl neo-ester-containing polymer only exhibited blistering around the scribe and that corrosion was confined to the scribe. On the other hand, the styrene acrylic primer shows very severe blistering and corrosion over the entire surface of the panel.
Exterior Wood Coatings
Without some type of protective surface coating, most woods deteriorate very rapidly during outdoor exposure. In addition to excellent resistance to weathering and UV, other features such as good adhesion to wood, protection against liquid water ingress and a good balance between hardness and flexibility are key requirements for exterior wood coatings. Moisture protection, however, remains a critical parameter still to be optimized in the field of waterborne acrylic wood coatings.
Self-crosslinkable core/shell acrylic latices with a fixed core Tg of 54 °C and shell Tg of 0 °C were prepared, characterized and formulated into wood stains. The systems had a weight ratio of hard to soft material fixed at 30/70. The shells were composed of butyl acrylate, methyl methacrylate, acrylic acid, a wet adhesion-promoting monomer, and either vinyl neodecanoate or 2-ethyl hexyl acrylate as hydrophobic monomers at a level of 30 wt. % on total monomer.
Weathering performance of the wood stains was evaluated with an accelerated weathering test according to EN 927-6. Performance of the wood stains was also assessed by means of an outdoor durability test. This entailed exposure to continental European weather conditions in Belgium for a period of three years (Figure 9).
After 2000 hrs, no blistering, cracking, flaking or chalking could be observed for the wood stain based on the vinyl neodecanoate-modified polymer. Severe chalking was observed for the wood stain containing 30% 2-EHA as the hydrophobic monomer. Also, after three years of outdoor exposure, the wood stain based on the vinyl neodecanoate-modified acrylic performed better than the 100%-acrylic polymer containing 2-EHA. The superior combination of UV stability with increased hydrophobicity conferred by the vinyl neodecanoate incorporated into the polymer backbone contributes to the improved weathering resistance of the coating.7
Vinyl Neo-Ester-Rich Modifying Polymers
One way to enhance the performance of paints based on acrylic emulsions is to add modest amounts of vinyl acetate-vinyl neo-ester copolymers that are rich in vinyl neo-ester monomer. This concept simplifies the improvement of paint performance, and one vinyl neo-ester-rich blending polymer could potentially be used to upgrade many different coatings. In addition to enhancing performance, the use of these vinyl neo-ester-rich copolymers in acrylic paint systems provides an opportunity to reduce the system cost.
To demonstrate the performance enhancements made possible by adding vinyl neo-ester-rich copolymers to coatings based on acrylic polymers, a vinyl neo-ester-rich copolymer containing 60% vinyl acetate and 40% vinyl neodecanoate was synthesized, along with a 100%-acrylic polymer based on methyl methacrylate and butyl acrylate. Mixtures of the vinyl neo-ester-rich copolymer and the all-acrylic polymer were then used to formulate high-quality, 42% PVC paints. These paints were tested for scrub resistance versus the same paint formulation containing only the 100%-acrylic binder and another paint using only the vinyl neodecanoate-rich copolymer as binder. It is evident from the scrub testing results (Figure 10) that the vinyl neodecanoate-rich copolymer blending resins dramatically increased the scrub resistance of the paints to which they were added. Additionally, the testing shows that the vinyl neodecanoate-rich blending resin used as a sole binder exhibits superior scrub resistance.
Conclusion
Copolymerization of the hydrophobic vinyl esters of branched neo-carboxylic acids with acrylic monomers significantly improves the performance of acrylic binders. Modifying acrylic polymers with vinyl neo-ester monomers is an easy process and yields polymers with exceptional performance characteristics including very high alkali and UV resistance, excellent durability and adhesion, and outstanding water repellence. These high-performance vinyl neo-ester monomer-modified acrylic polymers are ideal for a wide variety of very demanding coatings applications including metal, elastomeric and wood coatings, coatings for cementitious substrates, and high-performance decorative coatings. In addition, the modification of acrylic emulsions by blending with vinyl neo-ester-rich vinyl acetate copolymers enables a convenient route to enhancing coating performance while simultaneously providing a potential route for reducing overall system cost.
By Zegui Yan, Victor Arriaga, David Vanaken and Carl Cavallin, Hexion Inc., Stafford, TX
References
1 Koch, H. Production of Carboxylic Acids from Olefins. US patent 2,831,877, filed 17 March 1952.
2 Koch, Dr. H. Fette, Seifen, Anstrichmittel, Über neuere bei der Synthese verzweigter Carbonsäuren erzielte Ergebnisse,Vol 59, issue 7, pages 493-498, 1957.
3 Vandenzande, G.; Smith, O.; Basett, D. Vinyl Acetate Polymerization in Emulsion Polymerization and Emulsion Polymers, Peter A. Lovell, Wiley, 1997.
4 Scholten and Van Westrenen, W.J., Vinyl and Glycidyl Esters in Modern Binder Design, Paint Ink Int., Nov., (1991), p. 8.
5 Basett, D. Hydrophobic Coatings from Emulsion Polymers, Journal of Coatings Technology, January 2001, p. 43-55.
6 Decocq, F.; Slinckx, M.; Nootens, Dr. C. A New Technology for Environmentally Friendly and UV-Resistant Waterborne Anticorrosion Paints, Journal of Protective Coatings & Linings, June 2001, p.48-56.
7 Havaux, N.; Vanaken, D.; Simal, F. Paint & Coating Industry, October 2010.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!




