Effect of Different Surfactants on Emulsion Polymerization of a Vinyl-Acrylic Latex, Part Two

Davizro / iStock / Getty Images Plus, via Getty Images.
In last month’s “Formulating with Mike” article, we started talking about surfactant choice in the formulation of a vinyl-acrylic emulsion. From that article, here are the formulations of the emulsions that were made.
Materials and Methods
Surfactants, Vinyl-Acrylic Emulsions, and Paint Formulation
The surfactants used in the study and their main properties are summarized in Table 1.
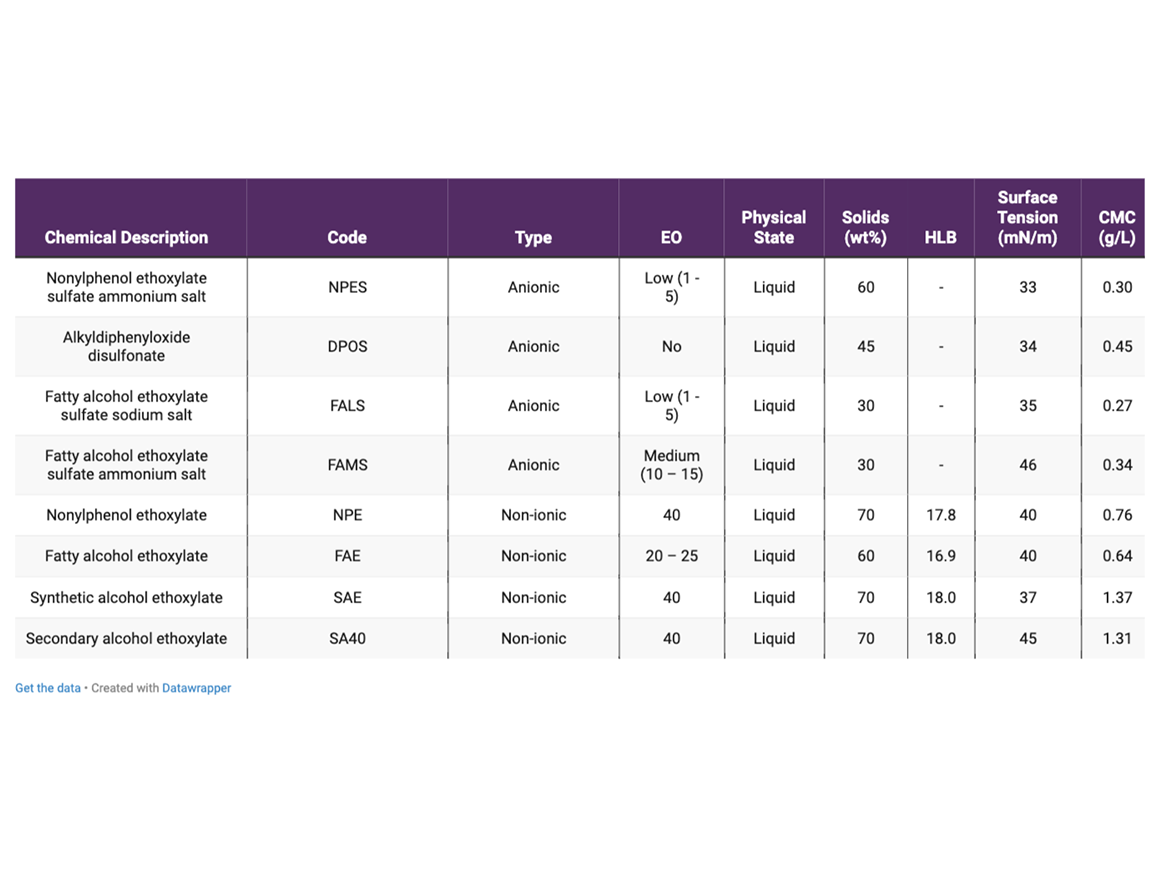
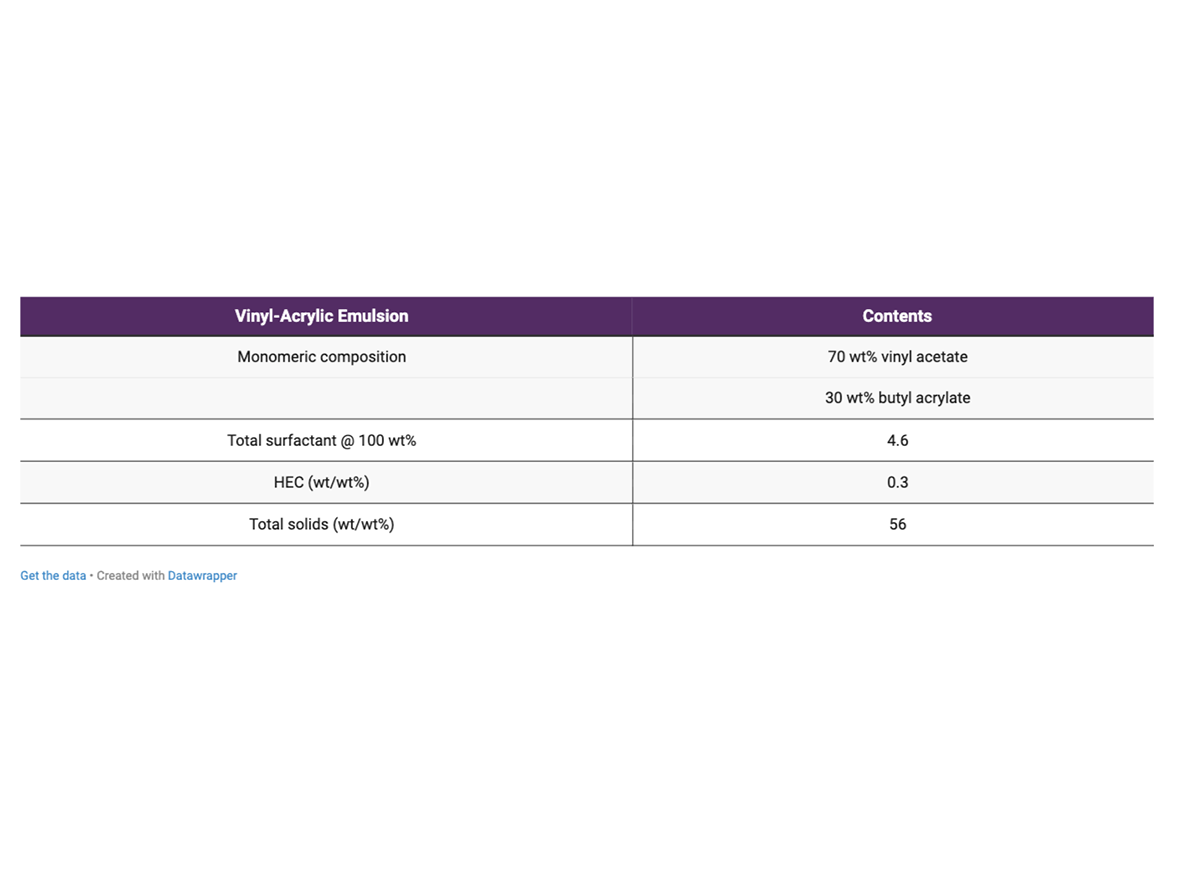
Table 2 contains the general formula for the vinyl-acrylic emulsion. The monomeric composition was chosen to generate latex with a calculated Tg of about 1 °C, so that it would require minimum coalescent, and as a result allow the development of formulations with VOC content lower than 50 g/L. The emulsions were synthesized in a 3 L reactor following a semi-continuous process under starve-fed conditions.
The compositions, processes, and raw materials used in this study were not changed during the assays. The only difference between the formulations is the type of surfactants used and the ratio between anionic to non-ionic surfactant. Table 3 contains the different surfactant compositions used in the study and the code for each formulation.

Results and Discussion
Considerations About Surfactants
The surfactants selected to be evaluated were chosen based on the criteria explained below:
- Anionic surfactants: Even though anionic surfactants do not play as important a role in vinyl-acrylic emulsion polymerization compared to polymerizations with more hydrophobic monomers, they are widely used to increase the electrostatic forces alongside the initiators to repel particles. The NPES was chosen because it is a well-known surfactant in the industry, even though regulatory restrictions are limiting its use. FALS and FAMS were selected because they are APE-free alternatives for NPES, and the goal is to evaluate how the change in the size of the poly(ethylene oxide) chain affects the performance of the anionic surfactant. DPOS was selected because it is a bulky anionic surfactant and does not contain poly(ethylene oxide) chains, so is different from the other three chemical surfactants.
- Non-ionic surfactants: Non-ionic surfactants are essential to guarantee stability of high-solid vinyl-acrylic emulsions. These surfactants adsorb on the resin surface and contribute to the steric repulsion between particles, avoiding coagulation or flocculation. All non-ionic surfactants selected have a high HLB due to their long poly(ethylene oxide) chain. The NPE was selected because it is a standard surfactant used in the industry, even though its usage is being restricted. FAE, SAE, and SA40 are all APE-free alternatives for NPE, and they were selected because they are based on different hydrophobes. FAE has a linear hydrophobe, SAE has a branched one, and SA40 is a linear hydrophobe but with a secondary hydroxyl group, which changes the conformation of the surfactant.
Vinyl-Acrylic Latex Characterization
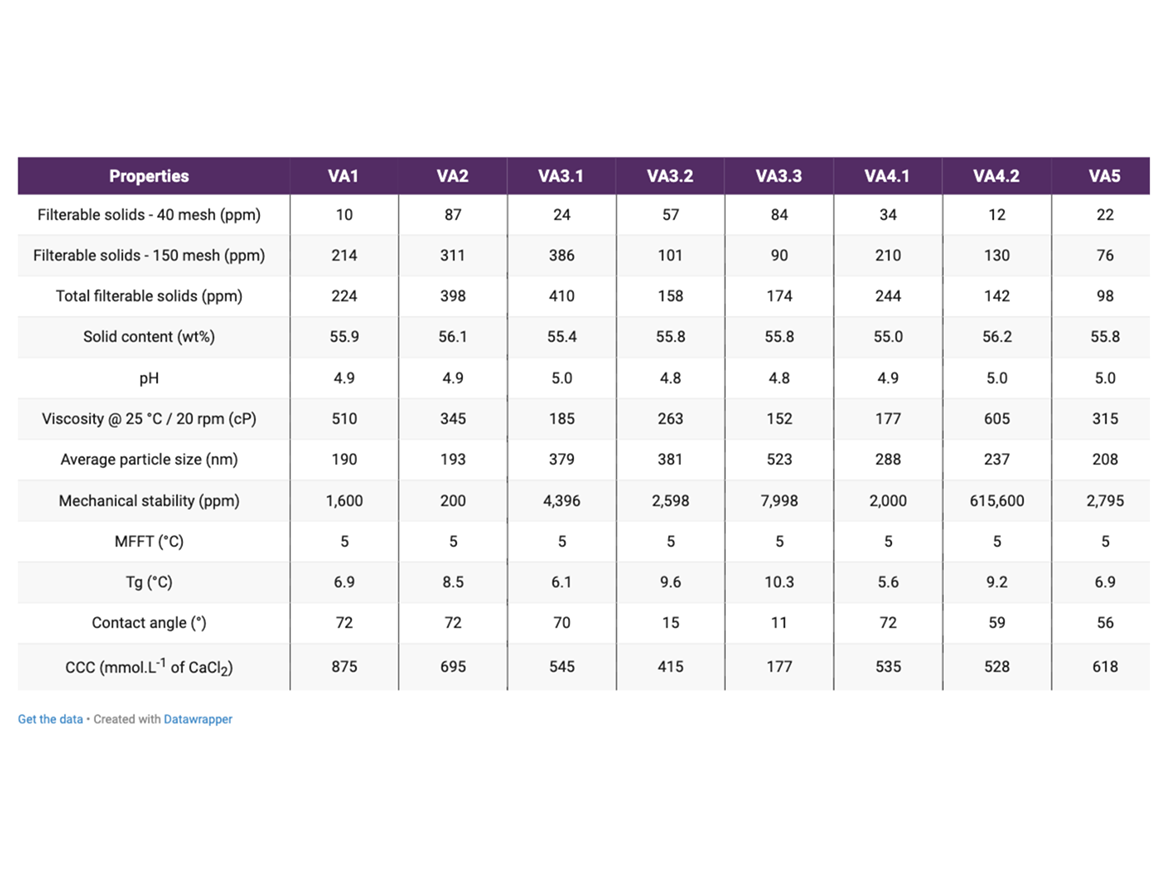
Table 4 contains the main results of the vinyl-acrylic emulsion characterization, and the following sections contain the discussion about some properties affected by the surfactant package selection.
Particle Size
Emulsion polymerization of vinyl-acrylic emulsions follows the mechanisms of homogeneous nucleation and coagulative nucleation due to the high solubility of the monomer-vinyl acetate-in-water and limited adsorption of conventional surfactants on particles of polar polymers. The coagulative nucleation mechanism allows a more suitable explanation for the particle growth along the polymerization and for the particle size distribution of the final vinyl-acrylic emulsions. The mechanism comprehends the following intervals:
- Interval I: Radicals are generated in the aqueous phase and cause the formation and growth of oligomeric radicals. They grow up until they lose solubility in the aqueous phase and precipitate, generating the precursor particles. Those precursor particles present high surface area and they coagulate, forming the primary particles. The particle size distribution of the primary particles depends on the colloidal stability of the system provided by anionic and non-ionic surfactants, protective colloids, and sulfate end groups that came from the initiator. The ionic strength of the aqueous phase also imparts the colloidal stability; the higher the ionic strength, the larger the particle size, and the broader the particle size distribution.
- Interval II: It is characterized by a constant number of primary particles, and the presence of monomer source. At this stage, there is mainly the growth of the primary particles swollen by monomers, and the new oligomeric radicals formed in the aqueous phase prefer to migrate into the primary particles swelled by monomers than to grow up in the aqueous phase.
- Interval III: The termination of the growing co-polymeric radicals inside of the emulsion particles.
As described above, it can be understood that the ability of the surfactant to adsorb on the surface of growing particles and to avoid massive flocculation or coagulation is one of the major events necessary to guarantee the colloidal stability of the emulsion and a narrow particle size distribution. Figure 1 contains the average particle sizes obtained for all the emulsions in this study, which were synthesized using the same initiator, same protective colloid, and same total surfactant content. Usually, particle sizes below 400 nm are acceptable for vinyl-acrylic latexes. The graph contains some trends that were further discussed.
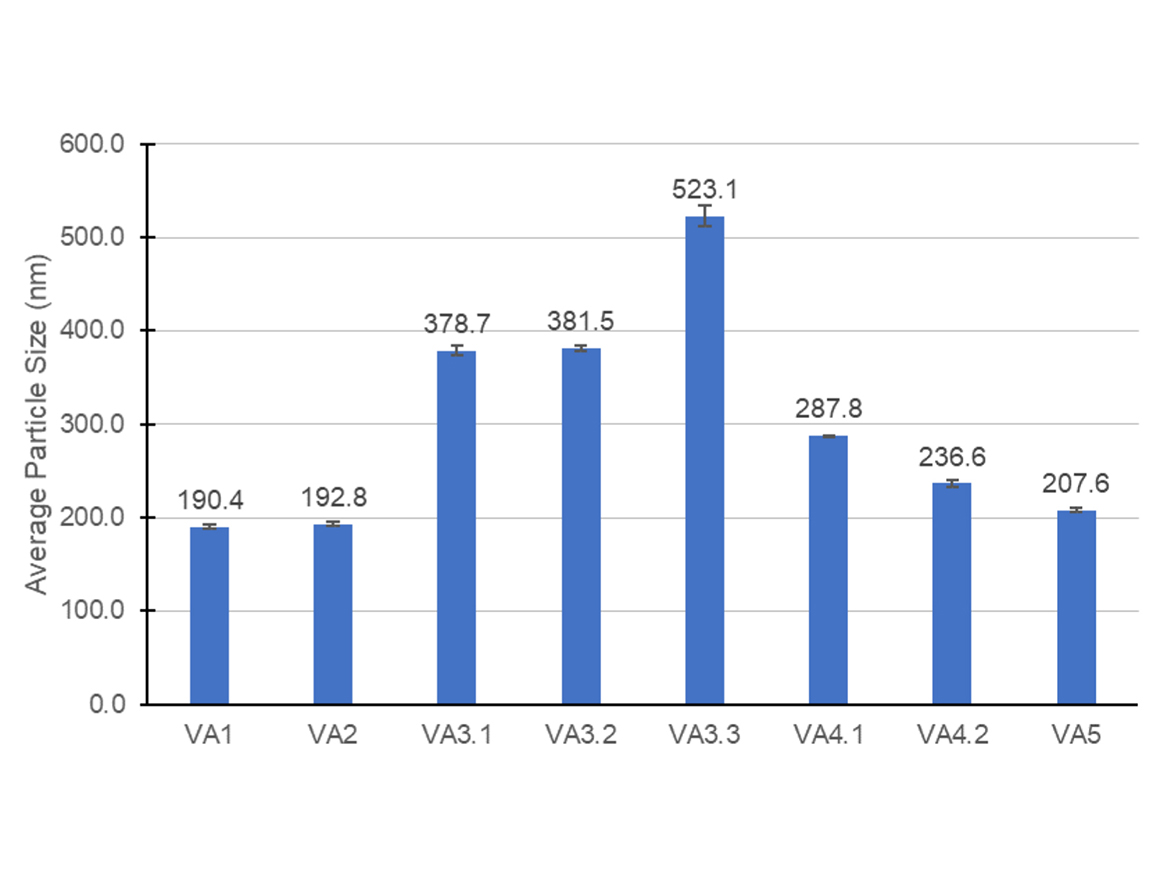
Latexes VA1 and VA2 presented the smallest particle size among all the emulsions prepared. This can be explained by a good coverage of the surface by the surfactants during the controlled coagulation of the growing particles. In the VA3 series of experiments, there is a slight trend of particle size increase as the amount of anionic surfactant expressively increased in the mix of surfactants. It can be assumed that the adsorption of the anionic surfactant on the surface of the particle was improved when the ratio was increased from 5 wt% to 15 wt%, since particle size was maintained, and filterable solids was reduced. However, further increase to 30 wt% of anionic content in the surfactant mix resulted in a bigger particle size. Possibly, the increase in the concentration of anionic surfactant was only increasing the ionic strength of the aqueous phase, shielding the charges in the surface of the particles, and then decreasing the electrostatic repulsion between them. This decrease of the electrostatic repulsion of the particles resulted in an increase of particle size.
The VA4 series of experiments resulted in a smaller particle size when compared against the VA3 series, and this outcome could be attributed to the higher poly(ethylene oxide) chain in the anionic surfactant which potentially increased the adsorption and stability of the emulsion particles. In this set of experiments, the increase in the ratio of anionic to non-ionic surfactant delivered a slightly smaller average particle size most likely related to a higher electrostatic repulsion of the growing particles. The VA5 differed from VA3.1 in the non-ionic surfactant used, which was more hydrophilic, and differed from VA4.1 in the anionic surfactant used, which had lower poly(ethylene oxide) chain. The result of the lowest particle size among these three experiments was interesting because it suggested that the stronger steric repulsion conferred by a more hydrophilic non-ionic surfactant such as SAE was more important in the control of the coagulative nucleation and particle growing of vinyl-acrylic latex than the amount of anionic surfactant and the electrostatic repulsion. Nevertheless, further studies are required to confirm this trend.
Filterable Solids
Coagulum and reactor scrap can be formed due to several causes such as low surface coverage from the surfactant (especially at higher temperatures), high electrolyte and particle concentration, flocculation of particles with highly hydrophobic segments, as well as many other causes. The most effective way to reduce coagulum formation (in this article called filterable solids), is to optimize the content of non-ionic surfactant, anionic surfactant and protective colloids, such as polyvinyl alcohol or hydroxyethyl cellulose (HEC), to increase the electrosteric stability of the emulsion particles during the polymerization. High-solid vinyl-acrylic emulsions are difficult to stabilize and APE surfactants are usually used to improve colloidal stability.
Figure 2 contains the total filterable solids obtained for all emulsions after the polymerization when the emulsions were filtered using 40-mesh and 150-mesh sieves (amount from both sieves added).
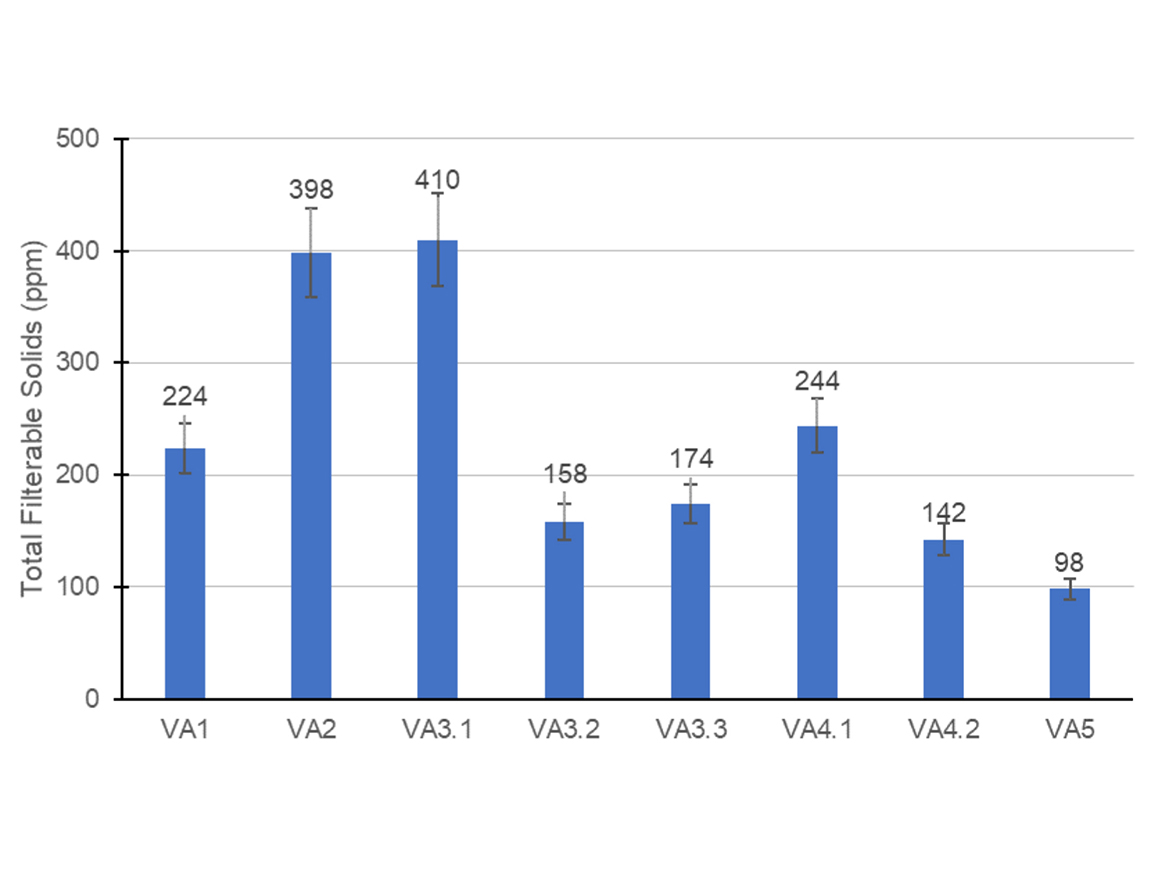
Usually, the acceptable amount of filterable solids formed during the emulsion polymerization of a vinyl-acrylic emulsion is up to 100 ppm filtered in a 40 mesh and up to 400 ppm filtered in a 150 mesh. The figure above shows that all surfactant systems evaluated avoided excessive coagulation during the polymerization. Some system tendencies are discussed below.
The emulsions prepared from formulations VA3 presented an interesting trend of coagulum formation. VA3.1 formed a similar amount of filterable solids compared to the APE-free formulation, VA2. The emulsions from formulations VA3.2 and VA3.3 formed less filterable solids than VA2, similar to VA1, which was the formulation with the APE-based surfactants. As mentioned before, particle nucleation and growth in vinyl-acrylic emulsions is controlled by partial coagulation of latex particles. These results showed that the stabilizing system used in the latex VA2 composed by sulfate from an initiator, sulfonate from anionic surfactant, and non-ionic surfactant with a 40 mol-poly(ethylene oxide) chain and HEC could stabilize the primary particles, and generate an emulsion with a small particle size. However, during the polymerization, this emulsion generated almost double the filterable solids in comparison to the APE-based formulation, VA1. This was probably caused by an unbalanced distribution of surfactants on the surface. Formulation VA3.1, despite having a bigger particle size in comparison to formulation VA2, also had a stabilizing system less effective than formulation VA1. However, formulations VA3.2 and VA3.3, with higher content of anionic surfactant, presented a more effective stabilizing system than formulation VA3.1, and even though particle size was higher, the filterable solids was lower than APE-based formulation VA1.
Increasing the content of the anionic surfactant in formulation VA4.2 also presented the same trend of decreasing the filterable solids in comparison to formulation VA4.1. Interestingly, though, the latex from formulation VA5, which contained the combination of the anionic surfactant used in formulation VA3.1 with a non-ionic surfactant with 40 mol of poly(ethylene oxide), generated the lowest coagulum formation in the reactor. These results were noteworthy because they showed that increasing the degree of ethoxylation of the non-ionic surfactant was more effective for decreasing the coagulum formation and decreasing the average particle size than increasing the content of anionic surfactant or replacing the type of anionic surfactant.
The VA5 formulation contained the anionic surfactant with a low poly(ethylene oxide) chain at the same concentration used in VA3.1, however, the filterable solids content was much lower in the first when compared to the latter. This was probably due to the higher electrosteric stabilization of particles generated in formulation VA5 using the non-ionic surfactant with 40 mol of poly(ethylene oxide), HEC and anionic groups from initiator, and anionic surfactant. Moreover, the more polar branched hydrophobe of the synthetic alcohol ethoxylate in comparison to linear fatty alcohol ethoxylate used in the formulation VA3.1 may have interacted better with the polar particles of vinyl-acrylic emulsion. These results reinforced that all surfactants can be used in the emulsion polymerization of vinyl-acrylic latex, but they must be balanced to achieve the appropriate stability and particle size.
Mechanical Stability
Besides particle size and filterable solids, the mechanical stability is another important evaluation to determine the stability of the emulsion, in this case, when exposed to a high shear, which can simulate industrial operations, such as pumping or blending with coatings components. Usually, emulsions with less than 10,000 ppm of filterable solids obtained after the mechanical stability test are acceptable. Figure 3 contains the filterable solids obtained after the mechanical stability test for all the latexes synthesized, except one.
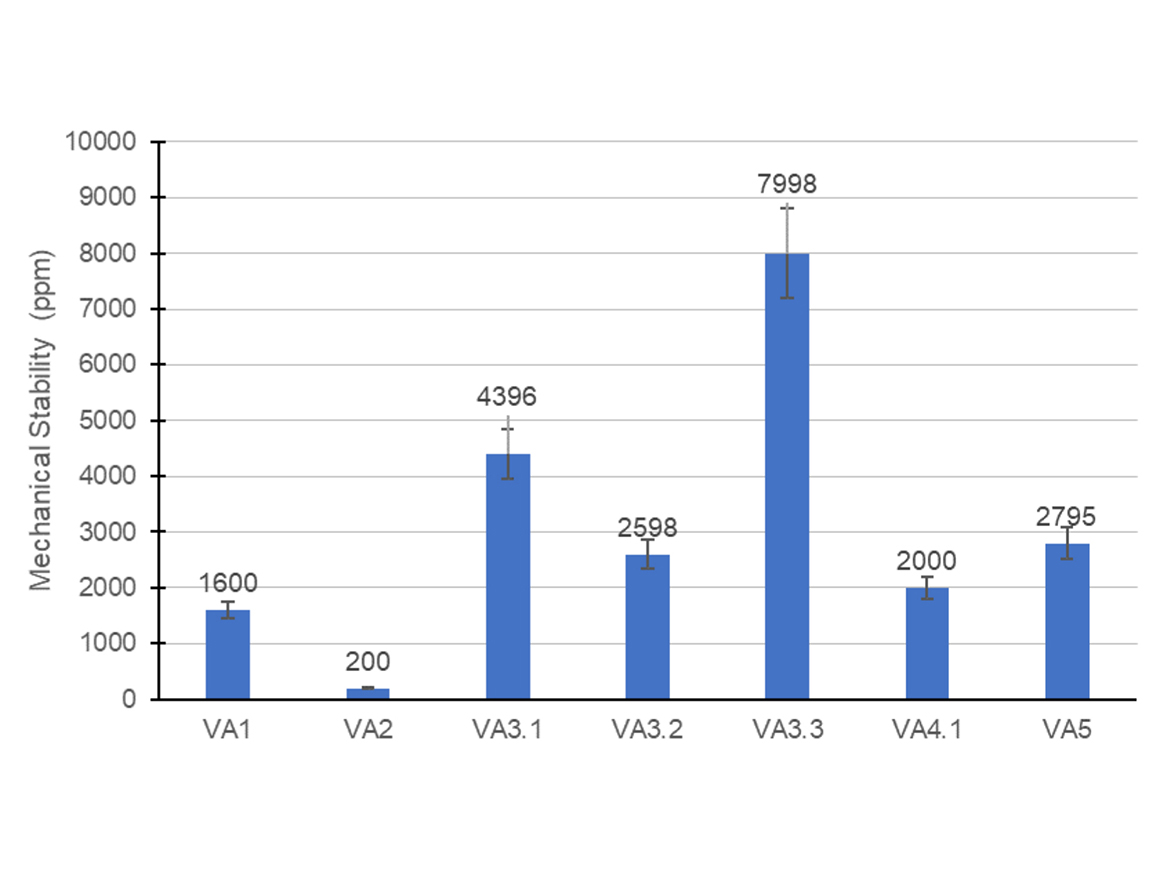
The VA4.2 formulation had filterable solids higher than the limit, hence it was not reported in this section. This result was curious because VA4.2 emulsion showed low filterable solids and small particle size, which led us to believe it had good stability. However, this failure in the mechanical stability test suggested that under shear stress, the lower steric repulsion caused by the reduction of the non-ionic surfactant content, resulted in the massive coagulation of the latex particles. On the other hand, the VA4.1 emulsion, which was richer in the FAE non-ionic surfactant, presented particularly good mechanical stability.
In addition to the hypothesis above, it could again be discussed that the increase in the concentration of anionic surfactant in formulation VA4.2 mainly increased the ionic strength of the aqueous phase, shielding the charges on the surface of the particles, and then decreasing the electrostatic repulsion between them. This decrease of the electrostatic repulsion of the particles resulted in a major coagulation when particles were forced together under shear stress, and hence caused a decrease of the mechanical stability of the latex.
Considering this, it would be expected that the VA3 series of tests delivered a lower mechanical stability when compared against the VA4 series, since they were synthesized with the same non-ionic surfactant, but the VA3 formulation contained the low-poly(ethylene oxide)-chain anionic surfactant. Nevertheless, the mechanical stability results for the VA3 formulations were acceptable and did not fail the test. A possible explanation to that lies behind the composition of surfactants on emulsion particles in the aqueous phase during the stability test that, for VA4 series, have possibly generated latex particles with low surfactant coverage and aqueous phase with high ionic strength.
The comparison between VA3 and VA4 series of tests was interesting because, for the same non-ionic surfactant, they showed how the anionic surfactant could play a significant role in the stabilization of the particles. These results were important to understand the ratio between different surfactants, and how their structures could be adjusted to optimize the stability of vinyl-acrylic emulsions.
It was also interesting to compare how changing the non-ionic surfactant could impact the stability of the system. The mechanical stability of latex VA5 was higher than VA3.1. A possible explanation lies in the type and structure of the non-ionic surfactant. VA5 was synthesized with a more hydrophilic surfactant with a longer poly(ethylene oxide) chain and a more-polar hydrophobe, which was probably creating better steric protection against coagulation. In addition, the more hydrophilic surfactant could possibly have a stronger adsorption to the particles surface, therefore presenting a lower level of desorption under shear stress.
VA1 and VA2 showed the highest mechanical stability among all latexes. VA1 formulation, APE-based, is known to deliver good stability due to the favorable adsorption of the surfactant on the particle surface. Formulation VA2 was synthesized with a DPOS surfactant, which presents a higher charge density per molecule and, therefore, it was possible to infer that there was a strong electrostatic repulsion contribution conferred by this surfactant. Additionally, the non-ionic surfactant used was very hydrophilic and was most likely adsorbed to the surface.
Electrolytic Stability
The increase in the ionic strength of the media shields the charges in the surface of the particles and reduces the electrostatic repulsion between them. The lower electrostatic repulsion favors coagulation of the particles that can be measured through the increase of particle size or the increase of the turbidity of the media. The higher the critical coagulation concentration (CCC), in mmol.L-1 of CaCl2, the higher the resistance of the emulsion to coagulate in a media with high ionic strength. In this scenario, the steric repulsion conferred by the non-ionic surfactants and protective colloids is the barrier for coagulation.
Figure 4 contains the CCC measured for all latexes synthesized. Overall, the CCC values for the vinyl-acrylic emulsions were very high when compared against other chemistries, such as styrene-acrylic emulsions. The analysis was conducted based on the particle size increase as the calcium-chloride solution was added to the emulsion, therefore, the trends observed are dependent on the initial particle size. The APE-based VA1 formulation showed the highest electrolytic stability among all emulsions prepared since it started from the lowest particle size obtained in the synthesis. Similarly, the VA2 latex also showed a high CCC, which was connected to the initial particle size being the second lowest.
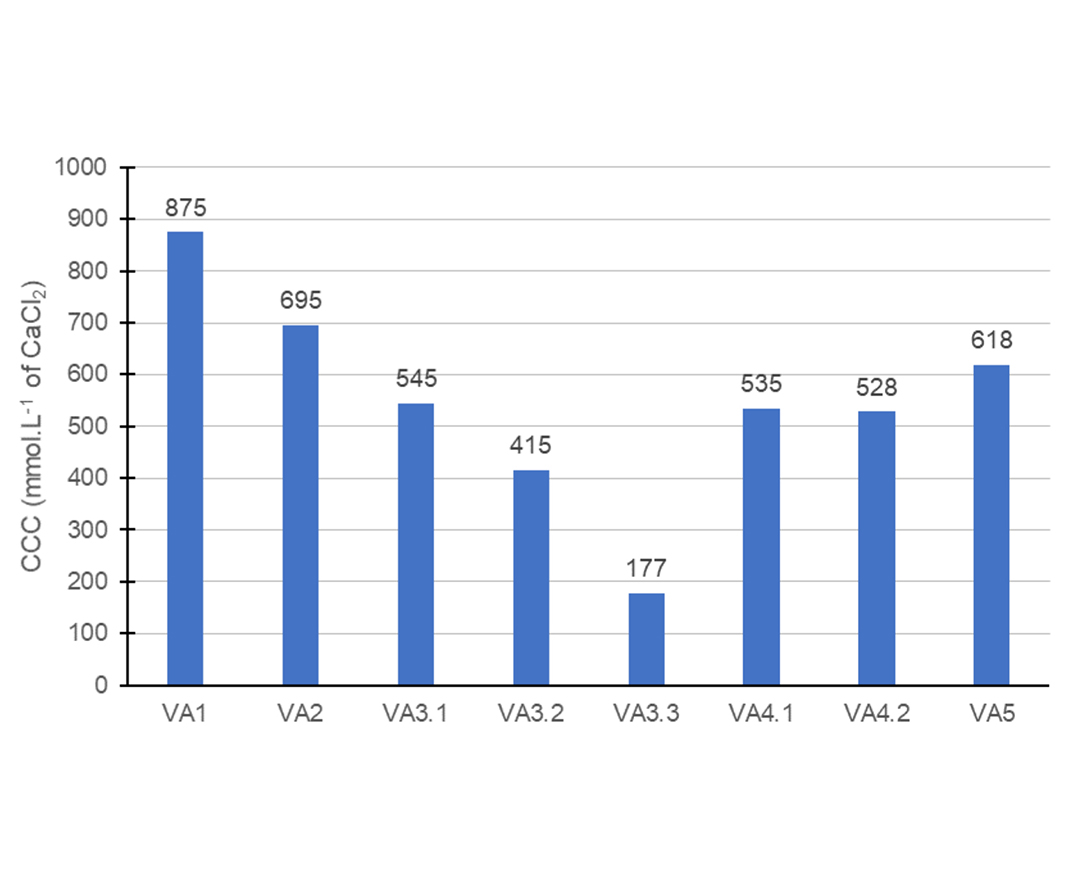
The VA3 series of tests showed an increased trend because even though it started from a high particle size, the CCCs are high. It is perceivable that the increase of anionic content in the mix of surfactants in the VA3 series caused a decrease in the electrolytic stability. One of the possible reasons why is the initial particle size that increased with the increase of anionic content in the mix of surfactants. In addition, the lower quantity of non-ionic surfactant covering the surface of the particles contributed to the reduction of electrolytic stability from VA3.1 to VA3.3. Overall, in terms of stability, latexes VA3.1 and VA3.2 were interesting candidates.
Comparing the VA3 with VA4 series, it was possible to see that the increase in the concentration of the FAMS anionic surfactant did not cause a reduction of electrolytic stability. It was an interesting result because the VA4.2 latex lost stability when exposed to high shear, but this formulation had presented low filterable solids and low particle size, failing only in mechanical stability. This result suggested that a possible improvement in the protective colloid used in the formulation could make it a viable alternative.
The comparison of VA5 with VA3.1 and VA4.1 showed that a more hydrophilic surfactant could improve electrolytic stability. The longer poly(ethylene oxide) chain on the surface of the particle could increase the steric barrier, avoiding coalescence even when the ionic strength of the media was high.
Glass Transition Temperature (Tg)
The synthesis of the latexes followed a semi-continuous batch process in a starve-fed condition to generate latexes with more homogeneous composition since the reactivity of vinyl acetate and butyl acrylate are quite different. The theoretical Tg of the latexes estimated, according to the Fox equation, was 1 °C. However, as shown in Figure 5, all latexes presented a Tg higher than 5 °C. This difference between theoretical and experimental Tg can be explained by the distribution of monomeric sequences and their impact on the stiffness of polymer chain.
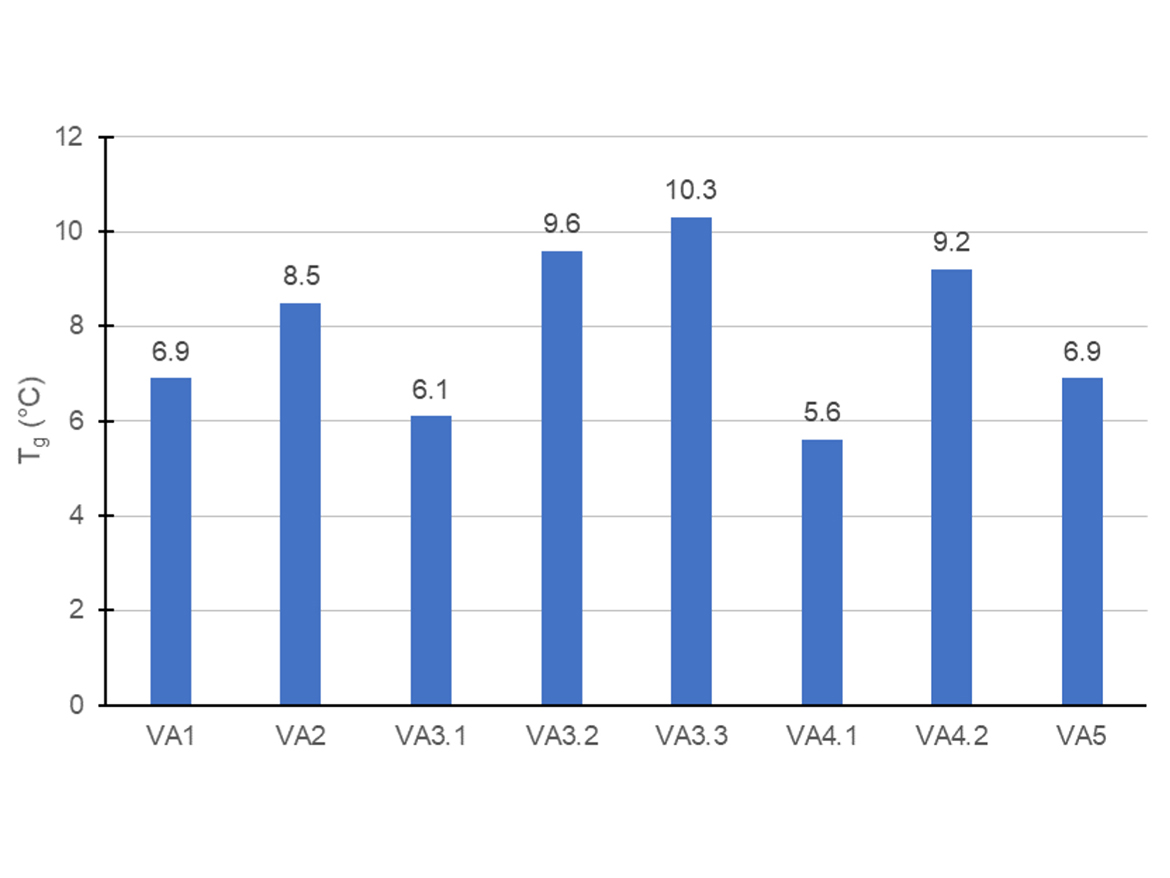
Interestingly, The VA3 and VA4 series showed that the increase in the anionic surfactant content in the mix of surfactants caused an increase in the Tg of the polymers. There were several possible explanations. One possibility was that the change in the composition of surfactants was affecting the solubility of vinyl acetate and butyl acrylate and, as a result, their copolymerization and microstructure of the final polymer chain. Another possibility was related to the plasticization of the latexes. It was possible that the higher concentration of non-ionic surfactant was favoring the plasticization of the polymer, hence reducing the glass transition temperature. Another explanation was that the increase in the content of anionic surfactants, or concentration of sulfonate/sulfate groups as in the case of VA2, was increasing dipolar interactions between polymeric chains and surfactants. It is possible that the copolymerization of a hydrophilic monomer with a hydrophobic one can be influenced by the concentration and composition of the anionic and non-ionic surfactant package as it affects the competition between homogeneous and heterogeneous nucleation.
Contact Angle
Water on a cleaned vinyl-acrylic film containing 70 wt% of vinyl acetate and 30 wt% of butyl acrylate presented a contact angle of 72° and estimated polarity of 0.23, close to the one poly(n-butyl acrylate) homopolymer, estimated to be around 0.21. This probably happens because of the re-orientation of polymer molecules richer in butyl acrylate, mainly located in the core of the latex, at polymer-air interface during the film formation. Figure 6 contains the results of the contact angle of water with the films of polymers synthesized.
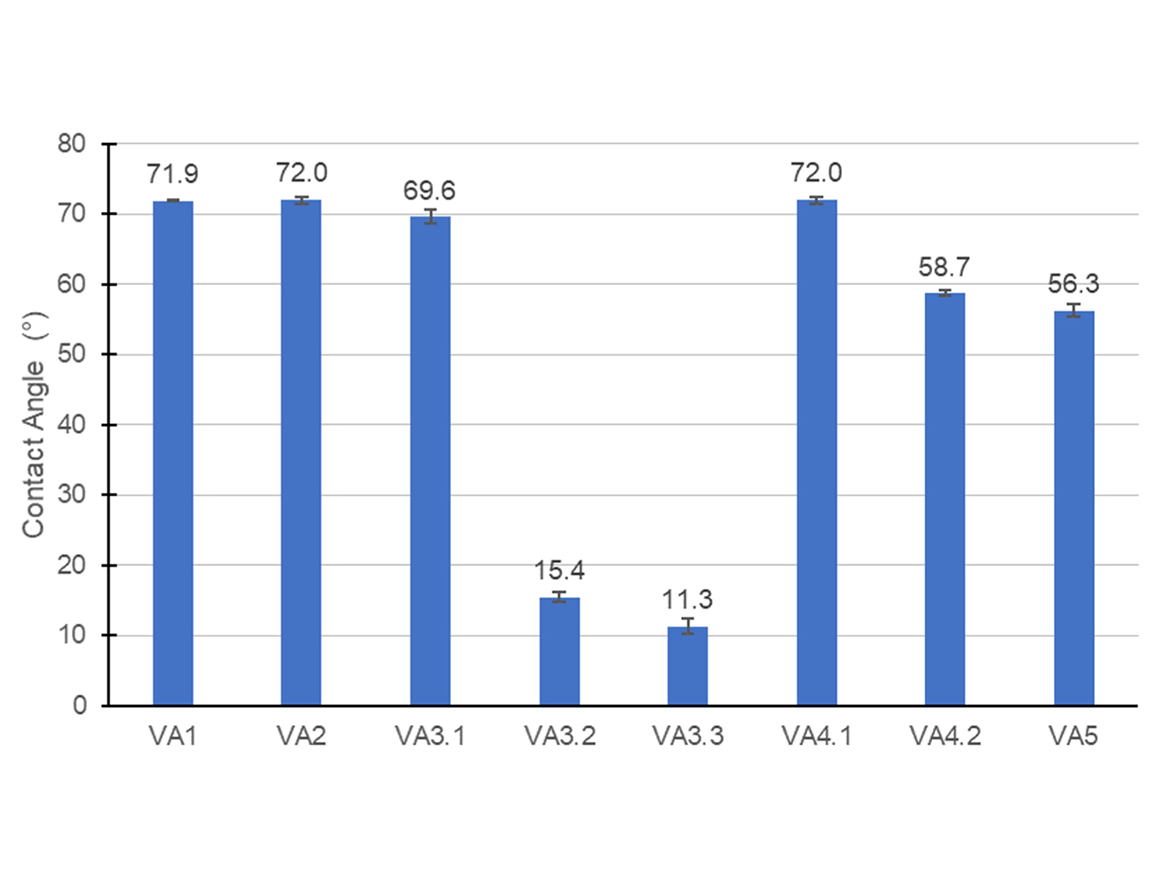
The results obtained with the VA3 and VA4 series were interesting, and they could be somehow related to the Tg results because polymer chains with specific microstructure or latex particles with different morphologies may present specific rearrangements of polymer chains and different distributions of surfactants at polymer-air interface.
In next month’s column we will show data on testing of paints made with these emulsions and cover conclusions drawn from this work.
All information contained herein is provided "as is" without any warranties, express or implied, and under no circumstances shall the author or Indorama be liable for any damages of any nature whatsoever resulting from the use or reliance upon such information. Nothing contained in this publication should be construed as a license under any intellectual property right of any entity, or as a suggestion, recommendation, or authorization to take any action that would infringe any patent. The term "Indorama" is used herein for convenience only, and refers to Indorama Ventures Oxides LLC, its direct and indirect affiliates, and their employees, officers, and directors.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!






