The emerging technical area referred to as “smart coatings” has stimulated a great deal of progress and debate. The debate centers around what exactly constitutes a smart coating. For this article, a smart coating will be a coating that can sense and respond to a stimulus in its environment (pressure, light, heat, etc.,) and react in a predictable and possibly reversible fashion. Variations on this definition will also be discussed.
The intent of this work is to discuss the definition of smart coatings and to give several examples of smart coatings. Materials that help in the design of smart coatings and some formulating strategies are discussed. An example of designing a practical smart coating using conventional materials is also covered.
Good general references to smart coatings are contained in the Smart Coatings series of books.1
Definitions
Many different ways of defining smart coatings have been published. One of these was discussed in the introductory comments. At the Smart Coatings III conference in February, 2011, Professor Jamil Baghdachi offered this definition: “Materials that are capable of adapting their properties dynamically to an external stimulus are called responsive, or smart. The term “smart coating” refers to the concept of coatings being able to sense the environment and make an appropriate response to that stimulus.”
An arguably better term for these coatings could be “functional” or “multifunctional” coatings, as proposed by this author and discussed by other authors.2
Is the smartness of a coating limited to its cured state? Smartness of a coating may also exist in the formation of a coating, rather than its cured state. In designing a coating, the chemical nature (i.e., surface energy differences) and the relative reaction rates of the coating ingredients may be selected so that the final, cured coating has a specific functionality at the surface. In extreme cases the formulation can self-stratify into separate functional layers.1
Regardless of definitions, the smart coatings field has exploded with new developments and materials. New materials for smart coatings are emerging constantly and are valuable additions to the formulators’ toolbox. Some of these are discussed in the next section.
Formulating Tools for Smart Coatings
Virtually any typical coating formulating ingredient can be used to make a smart coating if applied appropriately. However, certain materials have emerged recently that are particularly useful in providing increased functionality, often when used in relatively small amounts. Among these materials are those known as nanomaterials.
Nanomaterials are substances measuring below 100 nanometers (nm) in one dimension. Examples include:
- Carbon nanotubes; used for physical reinforcement, conductivity and other properties;
- Clays; also used for reinforcement, but also for barrier properties and for cost;
- UV screening particles; titanium, cerium and zinc oxides;
- Antimicrobial particles;
- Others.
Why Use Nanoparticles in Coatings?
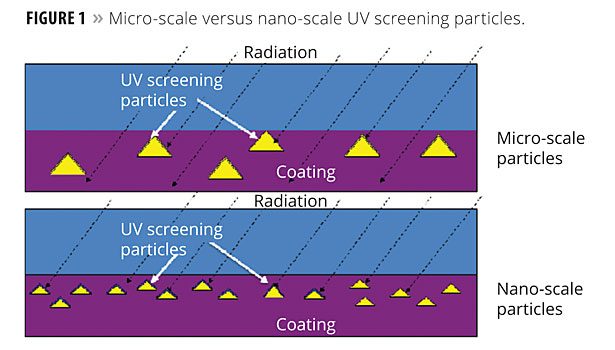
Actually, nanoparticles have always been used to formulate coatings, but sometimes without the awareness of their unique contributions to film properties. For example, nanoparticles may be dispersed more widely throughout a coatings film than micron-scale particles per unit weight. Figure 1 shows UV screening particles of two different sizes and their effectiveness at being positioned to block UV radiation.
Notice how the more numerous screening particles have a far greater likelihood of intercepting incident radiation due to their better dispersion throughout the film. Thus, nano-regime particles can be more effective than the same weight of micro-scale particles if they are sufficiently dispersed.
For UV absorption, metal oxide nanoparticles (ZnO and CeO2, etc.,) are particularly advantageous when compared to organic UV absorbers due to the combination of efficiency and lack of migration from the film. The organics are more efficient, but can migrate from the coating over time.
Nanoparticles can be more effective in providing other smart response properties as well. Conductivity, for example, is a critical function for coatings in a number of applications. Conventional conductive coatings generally require a large weight percent of conductive fillers to achieve high conductivity and tend to be opaque.
Properly dispersed high-conductivity carbon nanotubes (CNTs), however, can provide significant conductivity with the first few percent added. This, in turn, enables relatively transparent conductive films.
CNTs are basically cylinders of graphene. They are becoming increasingly available in both single-wall (SWNTs) and multi-wall (MWNTs) forms. Though they are still expensive, they represent a good current option for forming conductive transparent films.3
Graphene4 itself may hold even more promise, but is not as commercially available to date. Figure 2 shows an illustration of a SWNT.
Figure 3 shows the conductivity of MWNTs (also abbreviated as MWCNTs) as they are added into a polymer matrix. The high conductivity of SWNTs allows for a rapid increase in conductivity with only a few percent added to the polymer.
As shown in Figure 3, a small amount of MWNTs introduces conductivity into the polymer film. Because so little of the additive is needed, transparency can be 98% or higher. Applications include coatings for anti-ice applications on aircraft and other vehicle windshields.3
One key problem with the use of nanoparticles in coatings is the problem of dispersion. For carbon nanotubes the current method is to load a high amount of dispersants and subject the mix to high shear. Ultrasound is commonly used to provide the shear.
Dispersants can interfere with formulation stability and conductivity, however. During informal screening tests, the author has cast films of MWNTs that had no conductivity at all unless rinsed. The rinsing action removed dispersant materials, and the conductivity was greatly increased.
Recently, some vendors (such as InterNano) have announced dispersant-free CNT suspensions. These may help the commercial implementation of MWNTs and SWNTs significantly.
It should be noted that CNTs are not the only polymeric tools used to achieve transparent conductive coatings or to participate in anticorrosion functionality. Novel materials also include inherently conductive polymers (IHPs):
- Poly(3,4-ethylenedioxythiophene) oxidized with poly(4-styrenesulfonate) – PEDOT-PSS.:5 These formulations are currently used in waterborne inks.
- Polyanilines PAAs:6 these also tend to be waterborne formulations used in inks.
Both classes of materials may be useful as elements of smart coatings, but availability, expense and application issues are slowing their acceptance.
Other tools with smart attributes are now available to the formulator. These include:
- Infrared-reflective pigments for cooler car interiors and buildings;7
- Bio-based materials for cleaning the air of toxic materials and other functions;8
- Color-changing pigments and dyes (see, for example, solarcolordust.com), which can be used for security coatings, tamper evidence or freshness alert for food.
It is beyond the scope of this article to discuss nanomaterials beyond making the key point that new functionality has been made available or existing functionality has been greatly enhanced by nanoparticles. It can be argued that the term nanoparticle is simply semantics, but the counter argument is that the term has brought about a new awareness among formulators regarding particle size and its impact on properties; a beneficial development.
Smart Coatings for Corrosion Resistance
Corrosion is a huge problem, causing billions of dollars of damage per year. The most commonly used anticorrosion formulations of the last few decades have been based on chromium VI (CrVI).
Interestingly, the action mechanism of these very traditional CrVI coatings shares smart attributes. As illustrated in Figure 4, when a chromate-protected coating is damaged, the chromium migrates into the damaged area.
It is surprising to some that an “old fashioned” coating system fits into the modern concept of a smart coating, but the coating clearly responds in a predictable manner to an external stimulus to provide functionality (corrosion protection).
Smart or not, however, CrVI is essentially a banned substance worldwide. Novel approaches to corrosion protection are being researched extensively. There are far too many novel approaches to corrosion-control coatings than can be covered in this article, but a couple of examples are given below to illustrate some important ideas.
One particular strategy combines several methods, each one of which provides smart functionality. This strategy will be used as an illustration of smart coatings' anticorrosion possibilities. The illustrative strategy combines several smart functions: warning, corrosion inhibition and self-healing. The strategy is based upon incorporation of multifunctional micro capsules into a coating.9 The materials for these responses are encapsulated in a shell that is stable at neutral pH and hydrolyzable at basic pH, typical for corrosion processes. Figure 5 illustrates the structure of the functional microcapsules in this example.
The functionalities of this coating provided by the incorporated microcapsules per Figure 5 include:
- Detection and reporting (pH-sensitive shell and indicator);
- Galvanic response (corrosion inhibitor);
- Self-healing of damaged area (resin sealing).
Figure 6 shows how this approach could work in the case of actual damage to the coating by scratching.
When a scratch occurs on the coating’s surface, capsules are broken. The contents flow into the damaged area where the anticorrosion additive provides galvanic protection. The resin flows into the damaged area to provide physical protection after air-curing.
In the case of galvanic corrosion, the pH -sensitive shell hydrolyzes and the contents enter the damaged area to perform the protective functions described above.
Methods for making such capsules have been improving steadily, and methods such as this will likely be an important means of corrosion control in the next few years.10
The example in Figure 6 included a self-healing element as part of an anticorrosion package. Self-healing coatings themselves are a high-priority research topic at this time. This strategy is also being used to make self-healing composites and concretes.
Self-Healing Coatings
One approach to self-healing beyond encapsulants involves controlled reactivity. When a specially formulated coating is damaged, an external stimulation such as heat or radiation (i.e., UV) can be used to repair the damaged area.
Biswajit Ghosh and Marek W. Urban from the School of Polymers and High Performance at The University of Southern Mississippi, Hattiesburg, MS, have discovered a mechanism of self healing that incorporates some rather inexpensive materials.11
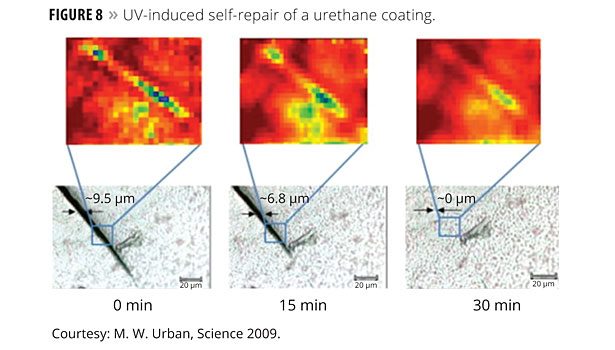
The authors report polyurethane networks that exhibit self-repairing characteristics upon exposure to ultraviolet light. The network consists of an oxetane-substituted chitosan precursor incorporated into a two-component polyurethane. Figure 7 illustrates a urethane-crosslinked network that is modified with oxetane groups (four member rings with oxygen as one member).
Upon mechanical damage of the network (network rupture), four-member oxetane rings open to create two reactive ends. When exposed to ultraviolet light, chitosan chain scission occurs, which forms crosslinks with the reactive oxetane ends, thus repairing the network. These materials are capable of repairing themselves in less than an hour and can be used in many coatings applications, ranging from transportation to packaging or fashion and biomedical industries. Figure 8 illustrates the resulting healing from this approach.
Chitosan is an inexpensive material and should enable a cost-effective technology to grow based upon this idea. The oxetane component is expensive, but might be replaceable by alternative reactive groups, depending upon future advances.
A similar healing took place with automotive lacquers (1960s-1970s). If a scratch occurred, lacquers could melt and re-flow to self-heal when heated. This was a huge advantage of lacquers over enamels, which, once crosslinked, could not flow. Only enamels, however, could meet VOC requirements that were established starting in the late 1970s.
Were these traditional lacquers smart? The author leaves that question to the reader for consideration!
Anti-Microbial Coatings
Other coatings are generally regarded as smart because of their antimicrobial properties. Some are and some aren’t, depending upon one’s definitions.
Generally, coatings are made to be antimicrobial by the use of additives. Antimicrobial additives for coatings and treatments can result in coatings that destroy microbes of various types. Examples of such materials include:
- Chitosan and the related “chitin” are mildly antimicrobial inherently. These materials can form antimicrobial coatings in and of themselves.
- Silver nanoparticles are highly antimicrobial and are used especially in textile treatments. There is some concern about the amount of silver nanoparticles being released into the environment during, for example, laundering.12
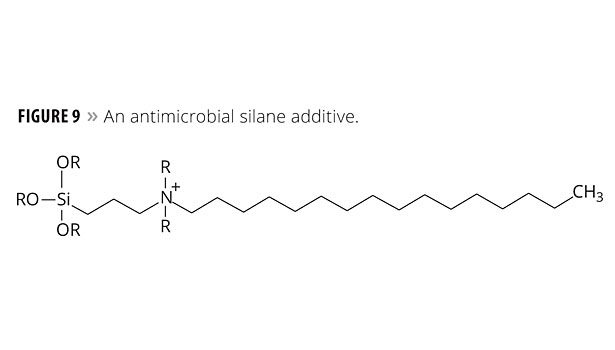
- Silanes – a particular class of silanes, discovered at Dow Corning, contains a quaternary ammonium salt and a long fatty “tail”.13
The chitosan and silver approaches are passive; the microbe happens to contact them and is destroyed.
The silanes, however, have a feature that attracts microbes, leading them to destruction. This active involvement may justify calling the silane coating a smart coating. An example of this class of silanes is illustrated in Figure 9.
In terms of mechanism, a microbe is attracted to the fatty “tail” and begins to ingest it. When the microbe cell wall contacts the polar ammonium center, the cell wall’s electronic structure is disrupted and the microbe dies. These silanes are now an Aegis Environmental Management product.
The Figure 9 material is in wide use throughout the consumer market in soaps, cleaners and surface coatings. The antimicrobial coatings derived from such silanes, and other materials, are also widely used in surfaces throughout the medical community, coatings tiles, grouts, ceramics, plastic tubing, etc.
So far, smart coatings have been discussed in terms of a single surface coating. Not all challenges can be resolved by single-layer coatings, however. Sometimes a series of coatings are used in combination to perform smart functions.
Multilayer Smart Coatings
Multilayer coatings, where each layer provides separate functionality, have been around for decades. The best known may be the basecoat/clearcoat topcoats for the transportation industry, where the basecoat provides aesthetics and the clearcoat provides protection.
More recently, smart multilayer coatings, layered functionality coatings, have been proposed for medical applications.14 For example, a smart coating “system” for medical implants has been proposed for hip replacement prosthetics, etc. The system is comprised of:
- Outer coating of pain killers for immediate post-operative comfort;
- Inner coating of antibiotics to maintain antimicrobial protection during the healing process;
- Innermost coating (against the implant) of growth factors to encourage complete healing.
One such system is illustrated in Figure 10, which illustrates the ordering of these functional films with respect to the direction of bone growth during the healing process.
The Figure 10 coating system is smart from the standpoint that the system supplies a specific need at a specific time in response to its environment, the healing tissue. All three layers are required to provide the overall result targeted.
When considering a coatings system to achieve a particularly complex smart result, it may be necessary to utilize multiple coats.
Self-Cleaning Coatings
Self-cleaning coatings cover a broad area that will be briefly summarized. These coatings interact with their ambient conditions in such a way as to keep their surface clean. Are they smart coatings? That depends on their design and use, and the readers’ interpretation of smart; they are certainly functional coatings.
There are two major categories of these coatings currently in use: hydrophilic and hydrophobic, water loving and water hating (or fearing). Hydrophilic coatings are coatings that have a contact angle with water of zero or near zero. Figure 11 shows a coating that is partly modified for hydrophobicity and shows the change in the contact angle of a water droplet from the left side (unmodified) to the right half (modified with a hydrophobic treatment).
Hydrophilic and hydrophobic behavior are due to two key factors: surface energy, a property inherent in the materials, and surface morphology, the micro and nano-scale structure of the surface.15 A high-energy surface will be driven to reduce the overall energy when coated with a low-energy liquid, driving the liquid to flow out, or have a low contact angle, relative to the surface. A low-energy surface has less need to minimize energy and, in fact, has less energy than most of the liquids wetting it. This surface tries to drive the liquid away, resulting in minimal contact with the surface, or “beading up”, as this behavior is generally described.
Hydrophobic materials in nature also have micron-scale protrusions from the surface and, in many cases, also have nano-scale protrusions upon the micro-scale protrusions.
Commercially, hydrophilic coatings are commonly comprised of metal oxides, especially titanium oxides. When such a surface is wetted by water, the water forms a smooth, transparent sheet over the surface, making access to the coated glass surface difficult. Additionally, TiO2 interacts with the UV from sunlight to form a species that degrades organic materials, further helping to clean the surface.
Hydrophobic coating technologies are more commonly used for self-cleaning applications. The concept is modeled after nature’s hydrophobic materials such as the lotus leaf and other leafy materials. As illustrated in Figure 11, hydrophobic coatings have a very high contact angle with water. That is, the water tends to bead up on the surface. The beads then tend to roll off the surface, picking up debris as they roll, thus cleaning the surface.
The smart coatings discussed in the section above are commercial or nearing commercial realization. In the next section, smart coatings under development are discussed, showing a selection of strategies that are being pursued for the next generation of smart coatings.
Smart Coatings Under Development
There are many smart coating technologies under development for commercialization in the five to 15-year timeline. To provide a sense of the technical direction being explored, the following strategies will be discussed:
- Superhydrophobic coatings;
- Stimulus responsive coatings (discussed in some detail);
- Others – listed as additional examples;
- Color changing;
- Air purification;
- Chemical agent resistant.
In the preceding section the topic of hydrophobic coatings was discussed. A topic related to hydrophobic coatings is superhydrophobic coatings.
Superhydrophobic Coatings
Thanks to new materials and equipment development, superhydrophobic coatings have become feasible and are being developed. These are coatings where the contact angle exceeds 150°.
Superhydrophobic coatings are in limited use and commercial availability. The uses tend to be in high-value applications such as electronics, aviation and fabric. The factors limiting application include:
- Superhydrophobic coatings’ surface morphology is sensitive to mechanical wear.
- Superhydrophobic methodologies typically demand expensive materials, special materials and/or application knowledge, or some special equipment and cannot be applied on all materials.
Possibly the most exciting aspect of the superhydro-phobic developments is that many of these coatings are also oleophobic, offering smart functionality that resists low-surface-energy materials as well as the high-surface-energy materials.
Stimulus-Responsive Coatings
Stimulus-responsive technology is surface technology that switches properties in response to a stimulus. The area is drawing a great deal of research attention.16 The technology grew from very exotic treatments of pristine gold surfaces studied in the 90s and is expanding into metals, plastics and fabrics. Application areas for surfaces modified to be stimulus-responsive include:
- Antimicrobial;
- Antifouling;
- Electrically switchable;
- Self-cleaning.
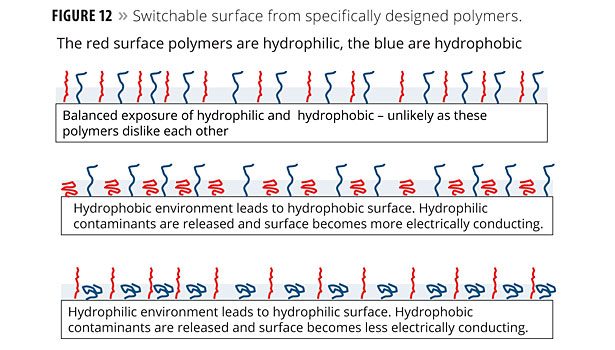
Figure 12 illustrates one model of how switchable surfaces are designed. The figure depicts a coating with polymers on the surface having different electrical properties (hydrophilic and hydrophobic). The red surface polymers are hydrophilic and the blue are hydrophobic.
The switchability mechanism illustrated in Figure 12 is driven by environmental pH. When the environmental polarity (pH) changes, the distribution of the exposed surface polymers likewise changes. Materials at the surface that cling to one set of polymers would be repelled or released when exposed to the second set. The idea of using functional polymers at the surface has broad applicability.
Example Development
This section discusses a recent product development that resulted in a smart adhesive coating for flexible membranes. The target model for the project was to produce a film with self-adhesive polymer brushes at the surface using currently available technology and useable on existing production equipment.
A client had need of a self-adhesive, printable coating that had good adhesion to a wide range of flexible membrane materials, generally organic films. The self-adhesive also had to be capable of medium (but reversible) adhesion to itself, with no significant adhesion to other materials. For commercialization, the self-adhesive had to have high-speed printing line compatibility and needed to be “moderate” viscosity at moderate temperature when applied.
The first step in designing a novel formulation is to carefully design a target model. For self-adhesion, the coating should be responsive to pressure, resulting in a bond that is neither too strong or too weak; that is, the self-adhesive strength needs to be high enough to stay sealed during normal handling, yet weak enough that a person with limited strength can still open it. The range of 300-600 grams per lineal inch (gpli) was chosen. To plan the path forward, the requirements for the final product were laid out and then planned backwards to determine the application performance and finally the raw materials required in order to achieve the ultimate objective.
To meet this target model, there were several requirements in the final, cured film:
- Very strong adhesion with excellent adhesion over a range of temperatures (for example, -50 °F to 100 °F);
- Sufficiently high Tg in the final, cured formulation to discourage adhesion to other materials;
- Perhaps a “polymer brush” structure at the surface. (Press the “bristles” together for adhesion.);
- Good balance of typical chemical resistance for food applications;
- Food packaging safe.
What application behavior will result in these final properties? The following list was generated:
- All major components must be inherently flexible;
- Adhesive components must migrate to substrate;
- Self-adhesive components must migrate to surface (functional self-organization);
- Suitable to current high-speed production methods;
- Radiation cure technology;
- Food-safe catalyst (UV cure);
- EB cure.
The resins that meet these criteria require specific molecular architectures. The model calls for a polymer brush surface. Such brushes can be synthesized, but that is expensive. It seemed that an in-situ formation of these brushes might be possible with the following structural elements and with controlled application.
One component (the adhesive component) would be selected based upon its excellent wetting to the target substrate. For example, if coating a polyester membrane, choose a polyester-like material. Designate this resin “X”.
A second component (the self-adhesive component) is needed with a long “tail” attached to a reactive group that can co-react with the first binder element. Designate this resin “Y”. Y must migrate to the surface so the “tails” can entangle when two coated surfaces are pressed together.
X and Y must form a stable, miscible blend. Miscible is likely better than soluble, since our model requires the components to separate prior to curing.
Then one must consider the functional self-organization in the wet material. This can be accomplished in two ways, or a combination of two ways: surface energy differentiation or reactivity differentiation.
Surface energy differentiation: If Y is to migrate to the surface, then the surface tension of X must be greater than Y. Any designed self-organization will require time to take place, therefore the manufacturing line layout must allow for sufficient wet time for Y to migrate to the surface after application of the product to the membrane. X will naturally migrate to the substrate, due to its chemical similarity as mentioned above.
Reactivity differentiation: Select reactivity where X>>Y. In a urethane system, for example, X could be a primary hydroxyl and Y a secondary hydroxyl species. For radiation cure, X could be acrylate and Y could be methacrylate, and so on. When cure initiates, the X components will rapidly cure at the substrate and the bulk with Y will be displaced and pushed toward the surface.
Other workers have reported self-stratifying coatings with sharp boundaries between the resin domains.1 In this example, the domain change will be more gradual.
Commercial resins were selected for testing and evaluated using design screens for application parameters, adhesion, self-adhesion, wet time requirement, cure speed and durability. Other testing was also run as the situation demanded, but will not be discussed here.
This work resulted in a final formulation and was identified with selective, or smart, adhesion to itself after the coating functionally self-organizes and is cured on-line. Patents are pending for this invention.
It is possible to make a smart process and product without resorting to new, exotic materials. Smartness can be designed in by looking holistically at the entire process from beginning to end by systematically building a model, and considering what responses are needed to produce desired results at each step. Thorough knowledge of the raw materials is needed; surface energy, reaction rates, etc., in order to assure that desired effects are realized and undesirable effects are minimized. Equally thorough knowledge of process engineering is needed; application method and conditions, wet time and conditions (sometimes called “flash time” or “dwell time”) prior to cure, and energetics of cure (how intense, duration, and when applied).
Conclusions
Smart coatings are an area of development and commercialization that promises to change the expectations of what can be accomplished with coatings. In the future, technology will be able to accomplish more functionality with less material due to the increasing efficiencies of the smart approaches. Several examples of such new technologies were discussed in this article. It should be noted that this “new” technological approach may be considered as much a shift in semantics as anything else. Some older coatings technologies possess properties most now would consider smart, such as the CrVI anti-corrosion mechanism or the lacquer self-cure.
This semantic shift is similar to the term nanotechnology. Nano-materials have always been present, but not separately recognized for the unique opportunities their small size offered. New semantics, even when applied to older materials or processes, are important in that they focus idea generation and awareness in ways not previously known or emphasized, enabling ideas that may not otherwise have occurred.
The definition of smart coatings is still evolving. The author would like to reinforce the “functional” viewpoint when discussing smart coatings, as covered in the beginning of this work. Also, one should consider the wet performance of a coating prior to set or cure as a potential arena for smart performance.
The example development presented in this article, where smartness was considered in every step in the development process, is one example of how a broadened perspective can improve the chances for commercial success using currently available (less costly) materials.
References
1 Baghdachi, J. and Provder, T. et. al., Smart Coatings III. 2011.
2 Challener, C. Smart Coatings - Getting Smarter? CoatingsTech2011, 8 (7), 26-31.
3 Wu, et. al., Transparent, Conductive Carbon Nanotube Films. Science2004, 27, 1273-1276.
4 Geim, A. K. Graphene: Status and Prospects. Science2009, 19, 1530-1534.
5 A.M. Nardes, et. al., Microscopic Understanding of the Anisotropic Conductivity of PEDOT:PSS Thin Films. Advanced Materials2007, 19 (9), 1196-1200.
6 Wesslinga, B. et. al. P., J. Corrosion prevention with an organic metal (polyaniline): corrosion test results Electrochimicha Acta1999, 44 (12), 2139-2147.
7 Synnefa, A., et. al. On the development, optical properties and thermal performance of cool colored coatings for the urban environment. Solar Energy81 (4), 968-981.
8 McDaniel, C. S.; McDaniel, J.; Wales, M. E.; Wild, J. R. Enzyme-based additives for paints and coatings. Progress in Organic Coatings2006, 55 (2), 182-188.
9 Calle, et. al. In A Multifunctional Coating for Autonomous Corrosion Control, Smart Coatings III, Orlando, Orlando, 2011.
10 Ghosh, S. K., Functional coatings: by polymer microencapsulation. Vch Verlagsgesellschaft Mbh: 2006.
11 Ghosh, S. K. et. al. Urban, M. W. Self-repairing oxetane-substituted chitosan polyurethane networks. 2009, 323, 1458-1460.
12 Nowack, N. C. M. et. al. B. Exposure Modeling of Engineered Nanoparticles in the Environment. Environ. Sci. Technol., 2008, 42 (12), 4447–4453.
13 Abbott, E. A. et. al. I., A. J. Method of inhibiting growth of bacteria and fungi using organosilicon amines. 3794736, 1974.
14 Roger J. Narayan, L. W. H. Chunming Jin and Afsaneh Rabiei, The use of functionally gradient materials in medicine, JOM Journal of the Minerals, Metals and Materials Society58 (7), 52-56.
15 Israelachvili, J. N. Intermolecular and Surface Forces, Third Edition. 3 ed.; Academic Press.
16 Urban, M. W. Stimuli-Responsive Polymeric Films and Coatings. American Chemical Society: Washington, DC, 2005.













Report Abusive Comment