Epoxy resin compositions have been employed for a variety of uses, such as laminating, coating, adhesion, encapsulating, molding, etc. In some uses, the polymeric materials are exposed to high temperatures that reduce the film integrity. A blend of an epoxy resin, a curing agent for epoxy resins, an organo-functional alkoxy silane and a catalyst for effecting condensation polymerization of a silane compound was found to provide high heat resistance and excellent mechanical strength.2 Similarly, complete or partial hydrolysis of alkyl/phenyl alkoxy silanes to form silanol or alkoxy-functional siloxane resins and the subsequent reaction with epoxy resins have been shown to produce copolymers with improved water and moisture resistance.3,4 The formulator must balance the desired property improvements with the cold-blend compatibility between the epoxy resin and the siloxane. Bis-phenol A epoxies have limited acceptance of silicones. Aliphatic epoxies are more tolerant of silicones, whereas cycloaliphatic epoxy systems are very compatible with silicones. Traditional modification of epoxy resins with SBT resins relies on cold blend mixtures with the potential reaction between silanol (SiOH) or alkoxy (SiOR) with available hydroxyl groups on the epoxies. This reaction, however, is not chemically favored. The Si-bonded reactive groups are more likely to homopolymerize rather than react with the epoxy. Any co-reaction is minimal and results in the formation of Si-O-C bonds. While these Si-O-C bonds are somewhat sterically hindered within the final resin matrix, they are ultimately hydrolyzable - creating a potentially weak link in the resin matrix. However, this copolymer form of silicone-epoxy bonds is widely utilized in the industry.
Other formulators have sought to ensure stable bonds between the epoxy and the silicone resin by utilizing the functional groups available from silane monomers. Resin formulators have created organofunctional (e.g., epoxy and amine) silicone resins for epoxy resin modification.5,6 While the use of silicone resin modifiers exploits the stability of the SiO2 matrix, modification of epoxy resins with silicone fluids takes advantage of other physical properties of silicones, such as flexibility.
The chain length of telechelic (linear) siloxanes possessing terminal reactive groups impacts the function of the SBT in the epoxy matrix. Silicone fluids with shorter chain lengths are inherently more reactive and miscible with the epoxy resin. This allows for modification of the bulk properties of the epoxy matrix such as toughness7 and impact resistance.8,9 Formulating with siloxanes that have longer chain lengths decreases the miscibility in the epoxy, and the properties of the SBT are governed less by the terminal organo-functional groups and more by the polydimethylsiloxane portion of the molecule. In those cases, the SBT becomes more of a surface modifier, contributing lubricity, low surface energy and water repellency.10
Experimental
This study compares modifications of a bis-phenol A epoxy paint prepared on a Cowles blade dispersator. After reduction with methyl isobutyl ketone to 30 seconds/#4 Zahn cup, the coatings were modified with a variety of silicon-based technologies, including aminopropyltriethoxy silane, aminopropylmethyldiethoxy silane, a methoxy-terminated siloxane resin, silanol-terminated siloxane resin and a novel amine-terminated siloxane resin. The control paint was formulated at an amine hydrogen to epoxy ratio of 1:1 using a standard, cycloaliphatic amine curing agent. The modified coatings were formulated by incorporating the silicon-based materials at four levels ranging from approximately 3-35% of the binder system. For those silicon-based materials containing amine functionality, the level of organic crosslinker was reduced to maintain the 1:1 amine hydrogen to epoxy ratio. The level of organic crosslinker was maintained for the silicon-based materials that did not contain amine functionality. For all samples, the pigment to binder ratio was maintained at a 2:5 p/b ratio.
The samples were mixed for 30 minutes on a mixing wheel and then allowed a 20-minute gestation period before application. Six-mil and 10-mil drawdowns were made on chromated aluminum panels (AL-39 from Q-Panel Inc.) and allowed to ambient cure for 14-days prior to evaluation. After 14 days, each of the samples was evaluated for gloss, solvent and chemical resistance, hardness, flexibility, recoatability, QUV exposure, cross-hatch adhesion, and dry time.
Gloss
Sixty-degree gloss readings were taken on each of the samples per ASTM D 523-89. A glossmeter such as the BYK-Gardner Micro-Tri-gloss (Catalog #4522) was used. Five readings were measured and averaged for each coating and compared to the control paint gloss. The percent change in gloss was reported.Solvent Resistance
Coating solvent resistance was tested by following CTM 0831. Eight layers of cheesecloth were wrapped around the ball end of a 24-oz. ball peen hammer and secured with copper wire. The cheesecloth was then saturated with methyl ethyl ketone (MEK) and placed on the panel. No downward pressure was applied to the hammer. The hammer was then rubbed back and forth perpendicularly to the direction of the drawdown. A forward and reverse stroke is one double rub. Double rubs were performed until a breakthrough to the metal surface was noted or until 200 rubs had been completed.Chemical Resistance
A 4-cm filter paper was placed on the coated panel, saturated with five drops of a chemical and covered with a 4-cm watch glass. After 24 hours, the panel was rinsed with water and rated as follows:-
5 - No effect
4 - Slight color change or blisters
3 - Moderate color change or blisters, or 1-10% film loss
2 - Severe color change or blisters, or 11-30% film loss
1 - 31-75% film loss
0 - 76-100% film loss
Hardness
Coating hardness was evaluated using the pencil hardness test following ASTM D 3363. Coatings were rated by attempting to scratch the surface with drafting pencils of increasing lead hardness. Hardness was rated as the highest lead hardness that could not scratch through the coating.Recoatability
The first layer of paint was cured for two weeks and then the specimen was overcoated with the same paint formulation and the control paint formulation. The topcoat was allowed to cure overnight and the panels were rated based on the ability of the topcoat to wet the underlying film. The rating chart below was used for evaluation.- 5 - Excellent wetting and flow; no film defects such as craters and fisheyes
4 - Slight dewetting and/or edge crawling; minimal defects
3 - Moderate dewetting and/or edge crawling; minimal defects
2 - Extensive dewetting and/or edge crawling; few defects
1 - Extensive dewetting and/or edge crawling; numerous defects
0 - Severe dewetting and/or edge crawling; extensive defects
Flexibility
Coating flexibility was determined using the Mandrel Test (ASTM D 1737). Cured coating panels were placed over the mandrel with the uncoated side in contact with at least a 2-inch overhang on each side. Using steady pressure of the fingers, the panel was bent approximately 180 degrees around the mandrel. It was then examined for cracking. This test was repeated with various mandrels until the coating cracked. The flexibility was reported in inches based on the diameter of the mandrel.Adhesion
Adhesion was tested following ASTM D 3359. A cross-cut tool scribe was used to score the coating by making eleven parallel lines about one-inch long and then a second set of scribes perpendicular to the first set. Three inches of pressure sensitive tape (Permacell P-99) was applied to the score and smoothed with finger pressure. The tape was rapidly removed at an angle of 180 degrees. The adhesion was rated as a percentage of the scribed area that remained intact. This testing was done for both coating to substrate adhesion and for intercoat adhesion.Dry Time
Dry time was determined using Gardner Circular Dry Time. A 4-inch wide, 6-mil wet film thickness drawdown was made on a pane of glass. The Gardner Circular Dry Time Recorder was immediately positioned over the wet film and switched on. The Teflon stylus with 12-gram weight scribes a circle, and the results are evaluated after a complete rotation as follows:-
1) Set-to-touch: During the first stage of drying, the film is mobile and partially flows back onto the scribed channel. The film may be considered "set-to-touch" when the material no longer flows back and the stylus begins to leave a clear channel.
2) Surface-dry: When the Teflon stylus no longer leaves a clear channel but begins to rupture the dry upper layer of the curing film, the surface may be considered to be "dust-free."
3) Through-dry: Finally, when the Teflon stylus no longer ruptures or dents the film but moves freely upon the surface, the cross section of the film may be considered to have reached the "dry-hard" condition.

Results and Discussion
GlossSixty-degree gloss readings were measured for the coatings and compared to the gloss of the base paint. Figure 1 (page 61) shows the change in gloss upon the addition of the silicon-based technologies. There was minimal (<8%) change in all of the materials with the exception of the coatings that contained >30% silane. This change in gloss of the silane occurs because methoxy evaporates off as the coating cures, creating stress on the coating.
Chemical and Solvent Resistance
The chemical resistance was tested against five acids and two bases. The average rating is shown in Figure 2 (page 61). The silanol resin performed similarly to the control, while the other silicon-based materials showed a slight decrease in chemical resistance. This decrease was caused by the degradation of the coatings from chemical attack by glacial acetic acid. Solvent resistance was also tested on the coatings by performing double rubs using MEK. All coatings, including the control, exceeded 200 double rubs.
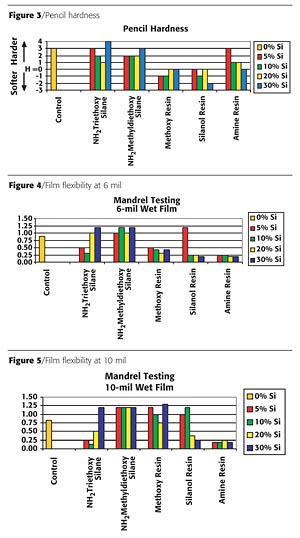
The pencil hardness of the coatings is shown in Figure 3 (page 62). Modification with the alkoxy silanes had minimal effect. However, the incorporation of the inorganic reactive (methoxy and silanol) silicone resins reduced the hardness significantly. This reduction was a direct result of the silicone resins not crosslinking into the system. The amine-functional silicone resin had a minor impact on the coating hardness with the low addition level (~4%) at "3H" and moderate impact at 36% loading with "H" hardness. Both readings are lower than the control sample at "4H."
Flexibility
Flexibility was tested at 6-mil and 10-mil wet film thicknesses. Figures 4 and 5 (page 62) illustrate that the amino-functional silane significantly improved the coating flexibility and that film thickness did not negatively impact coating flexibility. The methoxy and silanol resins improved the flexibility on the 6-mil coatings; however, the 10-mil coatings were significantly less flexible. The coatings containing silanes were very brittle and, therefore, did not improve flexibility.
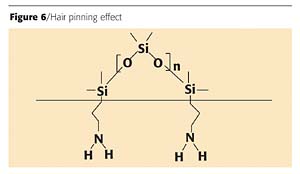
Linear organo-functional polymers improve flexibility. Although the amino groups bond into the system, thus providing flexibility, the silicone migrates to the surface of the coating and causes a hair pinning effect on the coating. This hair pinning, as illustrated in Figure 6 (page 64), creates the recoatability issues.
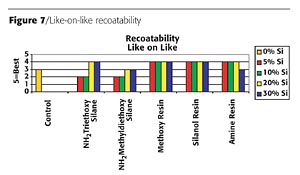
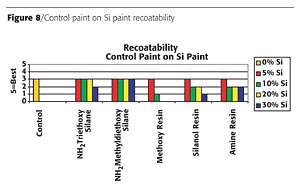
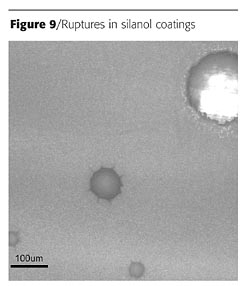
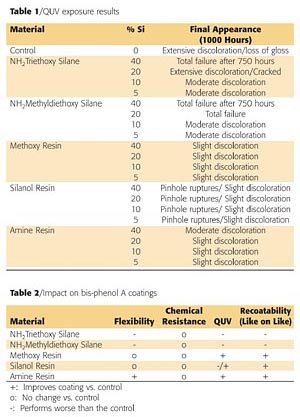
Panels of each formulation were placed in a QUV-B weatherometer for 1000 hours. Table 1 (page 66) shows a summary of the silicon-based technologies performance. Both silane technologies delaminated and crazed during exposure to the QUV-B. The control paint did not perform well upon exposure as it had severe discoloration and loss of gloss. The silicone resins all performed better than the control. The silanol resin appeared to have the best resistance to the UV exposure; however, after closer evaluation, pockets of pinholes were found in the coating. Figure 9 is a micrograph that shows the ruptured blisters over the surface and through the coating. These ruptures are likely due to the evolution of water during the condensation cure of the silanol resins at elevated temperatures. No other samples exhibited this defect.
Another important property of the epoxy paint that was monitored was coating adhesion. The adhesion test measured the coating-to-substrate adhesion and the intercoat adhesion of the two layers of coatings. All of the coatings containing the silicon-based technologies and the control coating appeared to have 100% intercoat and coating-to-substrate adhesion.
Dry Time
Dry time was tested to determine if the SBT had an impact on the cure time of the coatings. The test indicated that both the SBT coatings and the control coating were set to touch within 4 hours and thoroughly dry 6 hours after they were coated.
Conclusions
Bis-phenol A coatings currently provide good corrosion and chemical resistance; however, there are limitations such as flexibility and weather resistance. Current silicon-based materials used to modify bis-phenol A coatings provide shifts in coating performance, but create other issues, such as recoatability problems. The addition of silicone resins improves the overall performance of the coatings. The incorporation of the new amino-functional silicone resin provides the most significant improvement in flexibility at various thicknesses while having minimal impact on other aspects of the coatings performance. Table 2 (page 66) summarizes the impact of silicon-based technology on bis-phenol A coatings.References
1 Weiger, F.; German Patent. 1050051, February 5, 1959.2 Murata,Y., et al.; Shell Oil Company; US Patent 6005060 - Epoxy Resin Composition and Cured Composite Product; Dec. 21, 1999.
3 Mikami, R.; Toray Silicone Co. Ltd.; US Patent 4283513 - Siloxane-Modified Epoxy Resin Composition; Aug. 11, 1981.
4 Mikami, R.; Toray Silicone Co. Ltd.; US Patent 42837326 - Siloxane-Modified Epoxy Resin Composition; Aug. 11, 1981.
5 Decker, G., et al; Dow Corning Corp., Toray Industries; US Patent 5135993: High Modulus Silicones as Toughening Agents for Epoxy Resins; Aug. 4, 1992.
6 Witucki, G., et al; Dow Corning Corp; US Patent 5280098 - Epoxy-functional Silicone Resin; Jan. 18, 1994.
7 Ryang, H-S.; BASF Corporation; US Patent: 4847154; Thermosetting Resin Systems Containing Secondary Amine-terminated Siloxane Modifiers; July 11, 1989.
8 Keil, J.; Dow Corning Corp.; US Patent: 4624998 - Silicone-modified Epoxy Resins Having Improved Impact Resistance; Nov. 25, 1986.
9 Ito, H.; Takahashi, I.; Mitsubishi Denki Kabushiki Kaisha; US Patent: 5306747 - Flexibilizers of Hydroxyphenyl Silicone Oil-Epoxy Resin Product and Epoxy Silicone Oil-Phenol Resin Product; April 26, 1994.
10 Mikami, R.; Dow Corning Toray Silicone Co., Ltd.; US Patent 5364923 Organopolysiloxane Graft Epoxy Resins and a Method for the Preparation Thereof; Nov. 15, 1994.



Report Abusive Comment