
There are a number of basic ingredients necessary in formulating waterborne architectural paints - the latex resin (binder), pigments, coalescent solvents, thickeners and the additives. Among these additives are surfactants that offer dispersion stability, provide needed wetting for substrate application, and enhance characteristics such as gloss and color development. This paper addresses the effects of surfactants - phosphate ester-based surfactants in particular - in semigloss waterborne architectural formulations.

Surfactants and Phosphate Esters
Surfactants (derived from 'surface active agents') are chemical substances that, when present at low concentrations in a system, have the property of migrating and being absorbed on the interface, which is typically a water-oil or water-air type. The presence of these species in the boundary layer alters its interfacial free energies. Surfactants must be soluble in at least one of the phases in the system, and the substance must aggregate into micelles at certain concentrations. Typically, one of these phases involves water; therefore, the surfactant molecule must be amphiphillic - contain a hydrophobic tail and a hydrophilic head.Surfactants are often classified by the charge present in the hydrophilic head. Anionic (negatively charged) surfactants are the most commonly used type of surfactant, and they are low-cost with high efficiencies. They provide good wetting and stabilization of emulsion dispersions through electrostatic repulsion. Nonionic (no-charge) surfactants work through steric stabilization and generally provide poorer wetting with low efficiencies. They are compatible with other classes of surfactants, but their solubility in water is temperature-dependent. Cationic (positively charged) surfactants and Amphoteric (charge-dependent upon pH of media) are two classes of surfactants used in more limited coatings situations.
One type of anionic surfactant - phosphate ester - contains the phosphate moiety as the hydrophilic head. Phosphate ester surfactants are produced by the reaction of alcohols with an activated phosphoric acid derivative. Typically, phosphate ester commercial products are comprised of a mixture of monoester, diester, residual alcohol and residual phosphoric acid (Figure 1). The property of the final phosphate ester mixture is primarily defined by the starting alcohol as well as the composition of the four different species. Therefore, the property of the final phosphate ester mixture can be tailor-made by altering the alcohol used in the preparation, as well as controlling the ratio of the different components present in the final product. Phosphate esters are initially prepared in the free-acid form, but can be neutralized to the salt form using any base, including sodium hydroxide, potassium hydroxide, ammonium hydroxide or any organic amine.
The general application of phosphate ester in coatings is based upon its surface activity and, therefore, it serves primarily as a surface-active dispersant. The introduction of the phosphate moiety into the formulation as a wetting and dispersing agent significantly enhances the gloss and color acceptance property of the paint, reduces the viscosity build-up due to aging, improves surface wetting, as well as providing more stable dispersions. Certain phosphate esters in low-VOC formulations can replace glycols and other volatile reagents, which results in improved open time as well as enhanced freeze-thaw and heat stability. It is important to note that the effects of additives are formula-dependent and, therefore, will vary when the components in the formulations are altered.
The presence of the phosphate group imparts some interactions with metallic surfaces, thereby exhibiting some anti-corrosion and metal-adhesion characteristics. Phosphate ester surfactants have been found to successfully stabilize pigment dispersions, including reactive pigments such as iron oxides and zinc oxides. These additives have been used to prepare stable and very homogeneous dispersions of both organic and inorganic pigments used as universal colorants.
Typically, phosphate ester surfactants are added to waterborne formulations in very small amounts (generally 0.2 - 0.5 % based on solids) either in the grind or in the letdown. However, it has been observed that addition of phosphate esters at any time during the latex paint manufacturing process yields positive results. Therefore, much speculation and investigation has been devoted to the theory that the use of phosphate esters prior to the paint formulation stage (during the binder preparation as either the emulsifier, or post polymerization stabilizer) should not only benefit the final property of the paint, but also reduce the detrimental effects of any additional surfactants in the formulation that are not needed during the paint-formulation stage.
This paper describes the use of selected phosphate ester surfactants in a low-VOC (50 g/L) semigloss paint formulation. It explains the advantages presented by these surfactants and compares them with other surfactants in the same formulation.
Experimental Approach
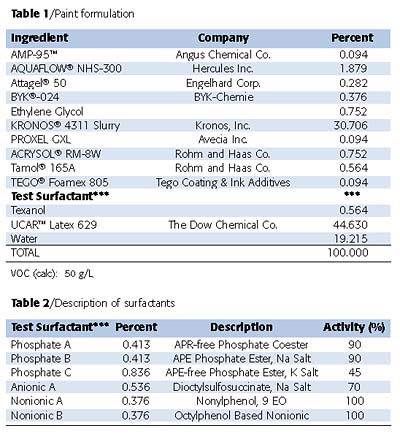
The paint formulation listed in Table 1 was prepared without the addition of the test surfactants listed in Table 2. The paint was divided into aliquots and the appropriate test surfactants added at a usage level of 0.4% surfactant based on the activity of the surfactant. The exact usage of each surfactant is also listed in Table 2. The resulting paint formulations were then mixed thoroughly before being subjected to a battery of commonly used architectural paint tests.Stability of the paint formulation was evaluated by determining both the KU and ICI viscosities at various times - as made, overnight and after one week. The paints were subjected to freeze-thaw cycles as well as heating to 140 °C for 10 days. Stability of the different paint formulations was evaluated under these extreme conditions by tracking the shift in viscosities.
The paints were evaluated for open time (using the wet edge brush out), surface wetting and also paint colorant stability - color rub-up and in-can color float. To simplify the analysis, only colorant B (carbon black) and colorant F (red iron oxide) were evaluated.
Film properties derived from these paint formulations were also evaluated for gloss (20°, 60°), contrast ratio, reflectance, block resistance, stain removal, yellowness index and flash rusting. Flash rusting was evaluated by drawing down a 4-mil-thick wet coating on an untreated cold rolled steel panel. The coating was allowed to dry, and was inspected 24 hours after application. The observed results have been rated using the standard set in ASTM D 610. All of the paint formulations were prepared and evaluated by Marschall Labs, Inc. (Clearwater, FL).

Results and Discussion
The control paint that does not contain any additional surfactant in the letdown was prepared and divided into aliquots. Appropriate amounts of surfactants as listed in Table 2 were added to the control paint. For comparison, the paint without any additional surfactant (Control) was also evaluated to see if any effects were evident when these additives were added to the formulation. These paint formulations were mixed thoroughly and evaluated for both in-can paint as well as cured paint film properties.Surfactants are typically added to paint formulations to enhance gloss, improve color acceptance and stability and promote surface wetting. Figures 2 and 3 describe the results on gloss evaluation. Upon any surfactant addition, gloss was enhanced. The non-phosphate ester additives gave a slight 1-2 unit improvement of the gloss for both 20° and 60°. When phosphate-based surfactants were added, a significant gloss improvement was observed - from a 5-15 unit increase. This suggests that the phosphate esters also serve as dispersion stabilizers - inhibiting flocculation. The addition of the phosphate esters in the letdown stage of the paint formulation offers this enhancement.
Surfactants also promote surface wetting. Figure 4 describes the effect of the different additives evaluated. Addition of the non-phosphate ester additives offers only slight enhancement on surface wetting, while phosphate esters show greater improvement.

Surfactants are useful in improving block resistance in waterborne paint formulations. The surfactant, like other blocking additives, rises to the surface of the coating, producing an oily, lubricating layer to prevent the two newly painted surfaces from sticking. This evaluation was done on films that were allowed to cure overnight. The test was carried out either at room temperature or at 120 °F. As shown in Figure 6, when evaluated at room temperature, phosphate esters provided only slightly better block resistance than the non-phosphate ester additives. However, when tested at 120 °F, only formulations containing the phosphate esters gave enhanced results. The non-phosphate ester products did not contribute to the block resistance of the paint.
In addition to blocking, surfactants are also used to promote stain removal by a similar mechanism. Figure 7 summarizes the results for stain removal. The phosphate ester surfactant gave improved results because of its high detergency effect compared to the other additives evaluated. This is a property for which phosphate esters are well known.

When different surfactants were added to the control paint formulations, the resulting KU and ICI viscosities of the paint were also altered (Figures 9 and 10). The KU viscosity relates to the bulk viscosity of the paint, while the ICI viscosity pertains to the viscosity of the paint during paint application (by roller or brush). Without any additional surfactants in the letdown (Control), the paint exhibited equilibrium KU viscosity of 102 and an ICI viscosity of 1.20. When Anionic A, Nonionic A or Nonionic B was added to the control paint, viscosity reduction was observed. This is actually a usual phenomenon and is due to the interaction between the additional surfactant and the thickener system present in the paint formulation. On the other hand, when any of the phosphate ester products were added, both the KU and ICI viscosities actually increased. This increase in viscosity offers the formulator the flexibility of retaining the amount of thickener needed in the formulation, or perhaps even reducing this level.
In addition to the properties mentioned, the formulations were also evaluated for other properties that will not be discussed in this paper. Properties such as scrub resistance, yellowness index, contrast ratio, reflectance, wet edge open time, heat stability, freeze/thaw stability and low-temperature film formation were not affected by addition of any surfactant. In addition, please note that this paper only compares the use of phosphate ester-based surfactants with other surfactants using a specific semi gloss latex paint formulation. The paint formulation was not enhanced to obtain optimum results for any of the properties evaluated.

Summary
The use of surfactant additives is necessary to promote gloss, increase color stability and improve substrate wetting. In the evaluation carried out in this paper, phosphate esters produced superior results in enhancement of gloss, promotion of color stability, increasing substrate wetting and improvement of stain removal. In addition, it was observed that only phosphate esters were successful in significantly improving block resistance. The presence of phosphate groups also resulted in enhancement of flash rust inhibition. The addition of phosphate esters to the paint formulation, unlike addition of other surfactant additives, does not reduce either the KU and ICI viscosities of the paint formulation.For more information, call 800/339.9111, visit www.dexterchem.com, or e-mail sales@dexterchem.com.
References
1 Flick, E. W. Water-Based Paint Formulations; William Andrews Publishing. Norwich, NY, 1998; Vol. 4.
2 Rosen, M. J. and Dahanayake, M. Industrial Utilization of Surfactants: Principles and Practices, AOCS Press. Champaign, IL, 2000.
3 Fay, T.; Sheridan, G. P.; and Karsa, D. R.; Comun. Jorn. Com. Esp. Deterg., Volume 12 (1981), pp. 295-309.
4 Koleske, J. V., Ed. Paint and Coating Testing Manual: 14th Edition of the Gardner-Sward Handbook, ASTM Press: Philadelphia, PA, 1995.






Report Abusive Comment