Compliance with increasingly stringent VOC legislation and an increased awareness of the importance of environmental stewardship have driven the development of many new low-VOC coating technologies. Waterborne acrylic polyols for 2K urethane finishes have played an important role in enabling regulatory compliance in the industrial and automotive finishes markets, yet their synthesis can be difficult. This article introduces a simplified method for the production of high-performance solvent-free waterborne acrylic polyols, made possible by Cardura™ E10P glycidyl ester technology. The performance characteristics of coatings made with these waterborne polyols is also demonstrated and compared to a commercially available waterborne polyol with significant solvent content.
The Birth of a Highly Versatile Family of Molecules
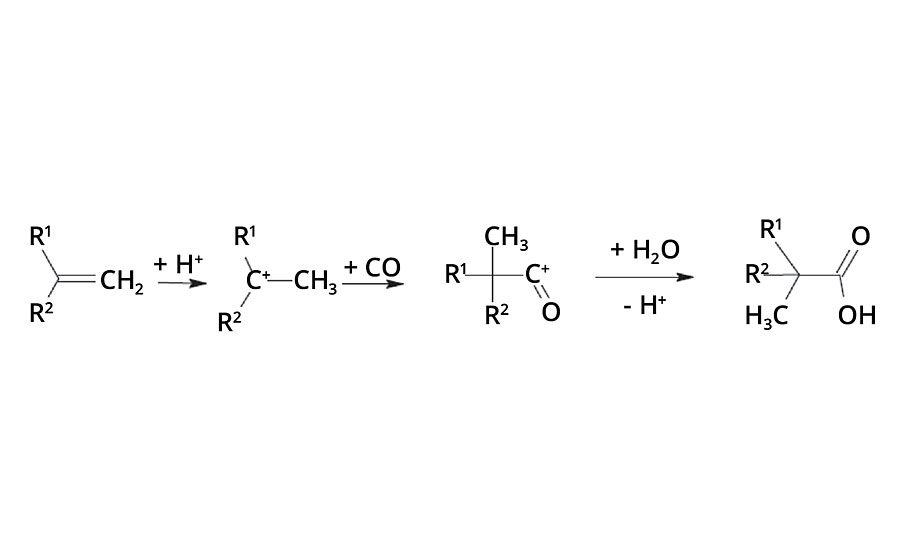
In the 1950s, Dr. Herbert Koch from the Max Plank Institute in Mülheim, Germany, found that olefins react with carbon monoxide and water under the influence of strong acids to form tertiary branched neocarboxylic acids (Figure 1). Before the intermediate carbocation reacts with carbon monoxide, isomerization reactions occur and, therefore, the resulting acid is composed of a number of isomers.1, 2
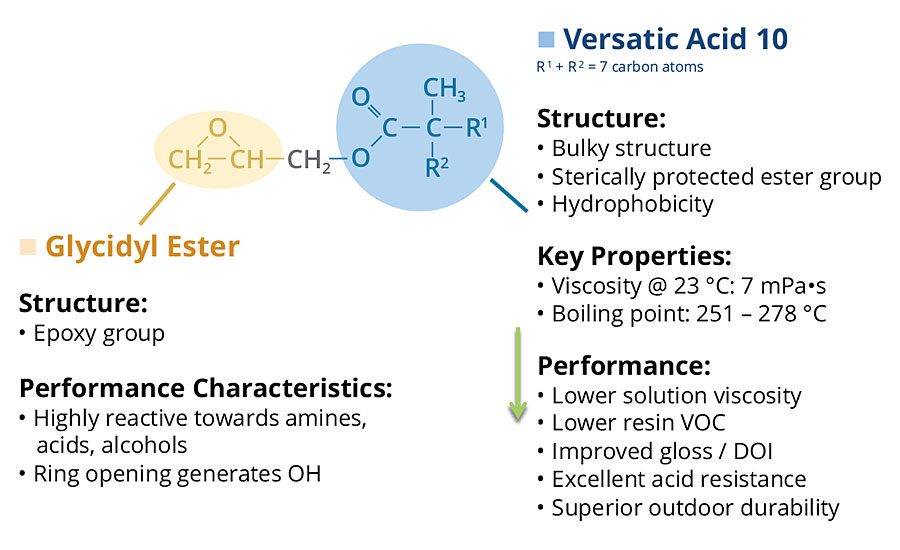
These neocarboxylic acids can be converted to their glycidyl esters by reaction with epichlorohydrin. Cardura E10P glycidyl ester is a particularly useful and commercially available glycidyl neoester manufactured by the glycidation of Versatic™ Acid 10, which is a neocarboxylic acid containing 10 carbon atoms (Figure 2).
Cardura E10P glycidyl ester is a highly versatile molecule containing a reactive epoxy group, and a very hydrophobic and highly branched tertiary-substituted a-carbon structure. The epoxy group is used to incorporate the molecule into polymers through reaction with carboxylic acid groups, and the neodecanoate group imparts outstanding performance characteristics. Incorporation of the glycidyl ester into polymers brings enhancements such as excellent acid resistance, superior wet look appearance, very good weatherability and low viscosity.
Conventional Synthesis of Waterborne Acrylic Polyols
Waterborne acrylic polyols (APOs) are typically prepared via a conventional radical polymerization process in solvent, much like their solventborne analogues (Figure 3). Waterborne APOs differ from solventborne APOs in that they contain a certain quantity of acid monomer, such as acrylic or methacrylic acid, to impart anionic character to the polymer. After the polymer is synthesized, these acid groups are neutralized with an amine to impart water dispersibility. One disadvantage of polyols prepared via the conventional process is their solvent content. A significant amount of solvent is required as a medium for the poly-mer-ization process, and without additional processing steps this solvent would remain in the polymer. To reduce the solvent content of the polymer to an acceptable level, an energy- and time-intensive distillation step is required.
Waterborne Acrylic Polyols with Cardura Glycidyl Ester
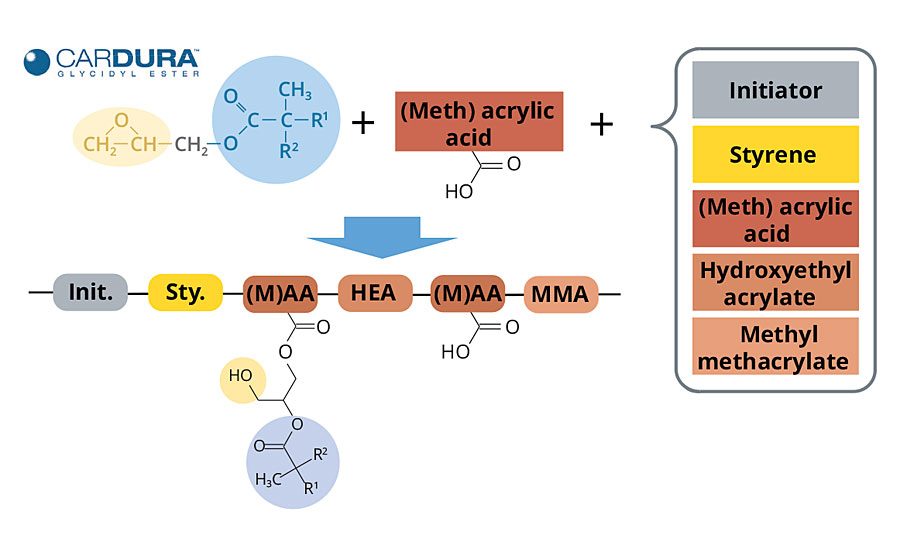
Cardura glycidyl ester has been used for some time as a reactive medium for solventborne acrylic polyol synthesis, and its value in enabling the easy synthesis of low-viscosity, high-performance, high-solids polymers is well documented.3-5 The synthetic pathway that this glycidyl ester provides to solventborne systems is greatly beneficial in waterborne acrylic polyol synthesis as well.
When used as a reactive medium for acrylic polyol synthesis, it replaces the solvent that would otherwise be required to conduct the polymerization. The glycidyl ester is gradually incorporated into the polymer backbone during the monomer feed step by the reaction of its epoxy functionality with acid groups contributed by acrylic or methacrylic acid present in the monomer feed. During this process, two reactions occur simultaneously: radical polymerization of the monomers, and the epoxy acid reaction that grafts the glycidyl ester to the acrylic polyol (Figure 4).
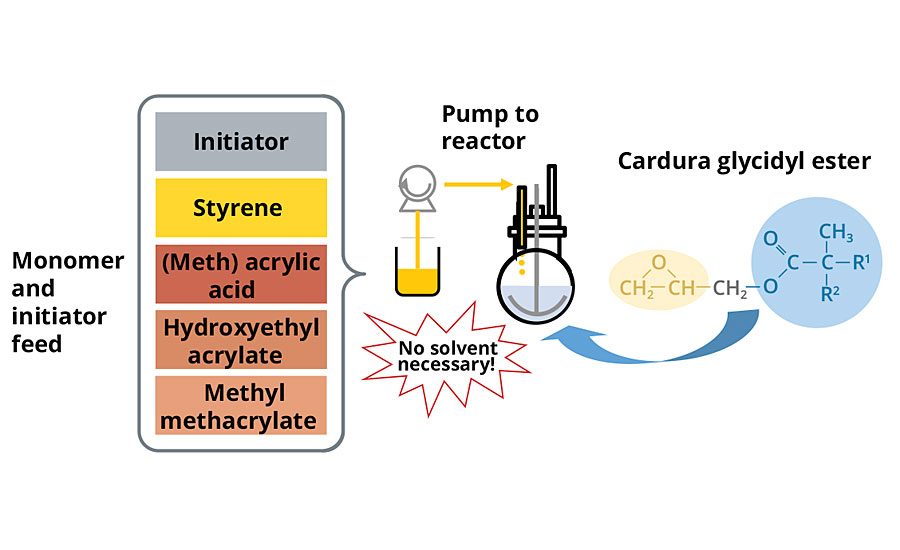
In a conventional waterborne acrylic polyol synthesis scheme, without special pressure reactors, processing temperature is limited to the boiling point of the process solvent. With the glycidyl ester as a process medium, solvents are not needed and the high boiling point of the material enables the high-temperature synthesis of acrylic polyols under atmospheric pressure. Additionally, since solvent is not necessary when using the glycidyl ester as a process medium, the need to conduct a solvent removal step to achieve low solvent content is eliminated (Figure 5).
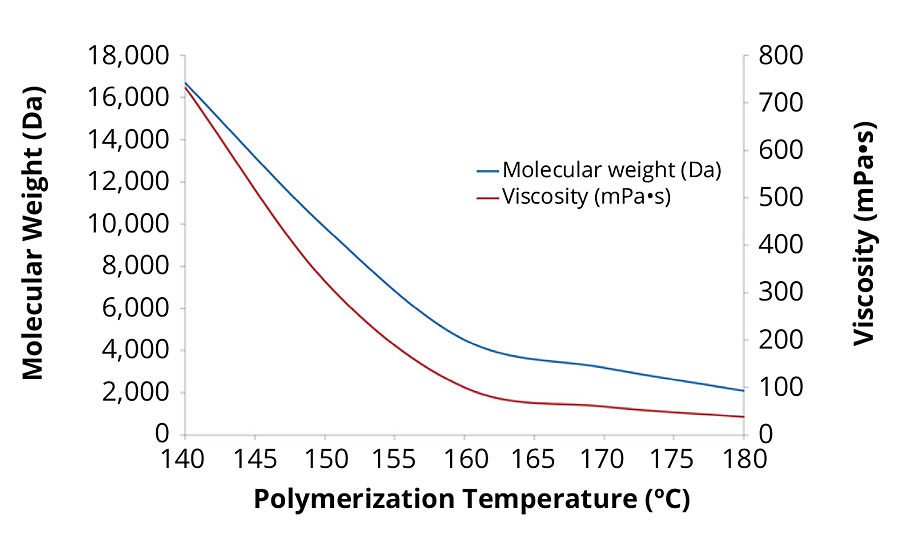
Synthesizing acrylic polyols at high temperatures is beneficial because it is a simple way to create low-molecular-weight, low-viscosity polymers without the need for large quantities of initiator or chain transfer agents, which can affect polymer performance (Figure 6). The low viscosity of the polyols synthesized with the glycidyl ester also makes the dispersion process easier.
Performance of Waterborne Acrylic Polyols Based on Cardura Glycidyl Ester
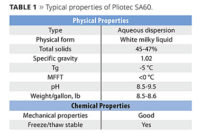
To demonstrate the performance of solvent-free waterborne acrylic polyols made with the glycidyl ester, a solvent-free polymer was designed with a Tg, hydroxyl number and acid value very similar to those of a commercial dispersion containing 12% solvent on polymer solids. These polymers were tested side by side in a 2K urethane clearcoat system and evaluated for a variety of film properties. The results demonstrate that Cardura glycidyl ester makes it possible to create solvent-free waterborne polyol dispersions that have performance on par with conventional solvent-containing waterborne polyol dispersions (Table 1).
Conclusion
Cardura E10P glycidyl ester enables the easy production of high-performance, solvent-free waterborne acrylic polyol dispersions. The use of this technology eliminates the need to distil solvent after production to achieve low VOC levels. Eliminating solvent removal from the process reduces cycle time, waste and production costs.
By Cédric Le Fevere de Ten Hove, Christophe Steinbrecher, Denis Heymans, Steven Mao, Marcelo Herszenhaut and Carl Cavallin, Hexion Inc., Stafford, TX
References
1 Koch, H. Production of Carboxylic Acids from Olefins, US patent 2,831,877, filed 17 March 1952.
2 Dr. Koch, H.; Fette; Seifen; Anstrichmittel. Über neuere bei der Synthese verzweigter Carbonsäuren erzielte Ergebnisse“, Vol 59, issue 7, pages 493-498, 1957.
3 Petit, P.; Henry, N.; Krebs, A.; Uytterhoeven, G.; de Jong, F. Progress in Organic Coatings, 43, 2001, 41-49.
4 Henry, N.; Moerman, M.; Uytterhoeven, G. Eurocoat, Lyon 1997.
5 Steinbrecher, C.; Le Fevere de Ten Hove, C.; Heymans, D. Hybridized Acrylic and Polyester Chemistries: High Performance Polyols for Solventborne and Waterborne Polyurethane Topcoats, at the Automotive Coatings conference, November 24, 2011, Berlin, Germany.








Report Abusive Comment