Waterborne Epoxy Zinc-Rich Primers: There Are Viable Options


In the specific case of epoxy primers enriched with zinc for extra corrosion resistance, the challenge of incorporating a very high weight (often 80-85%) of zinc powder into an epoxy/curing agent binder system is compounded by zinc’s reactivity with protic components such as water, acidic solvents and dispersing aids.
We have developed and tested several 2K waterborne epoxy/amine systems designed to improve pigment loading and to protect zinc from protic media that cause hazardous hydrogen generation. These new systems have been tested against classical solventborne and waterborne epoxy systems. The preferred system provides a storage-stable, 2K zinc-rich coating with a long pot life. It yields a flexible coating that provides steel with excellent corrosion protection at much lower VOC levels (~200 g/L) than solventborne systems. This article provides an overview of this novel 2K waterborne epoxy binder system that allows formulation with high levels of zinc.
Waterborne Epoxy Technologies Overview1,2
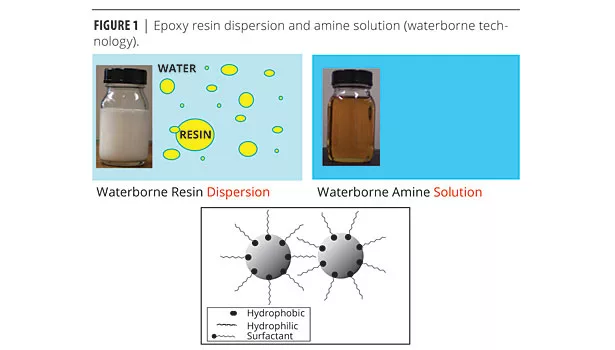
In early waterborne technologies, the epoxy resin was simply dispersed and stabilized in water (Figure 1). In more recent waterborne epoxy technologies, the dispersant and stabilizers are chemically reacted onto the backbone of the epoxy polymers rather than just blended with the resin. In both cases, the amine curing agent is usually a water-dilutable polymer.
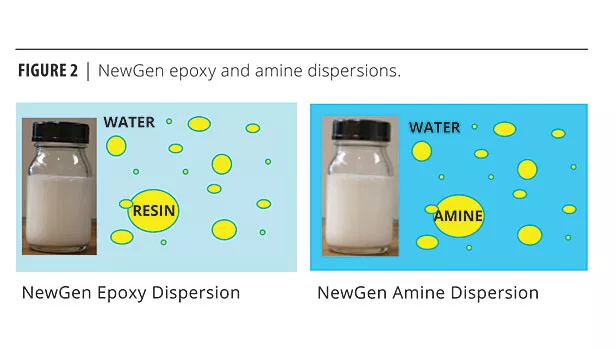
NewGen™ epoxy-amine waterborne technology (Figure 2) was designed to replace solventborne epoxy-polyamide coating technology. The epoxy (EPI-REZ™ Resin 6520-WH-53) is self-emulsified, contains no free surfactants, and is crosslinked with a unique, zero-VOC amine curing agent dispersion (EPIKURE™ Curing Agent 6870-W-53). This NewGen system allows formulation of fast-drying, highly corrosion resistant, <100 g/L VOC, ambient-cure primers and direct-to-metal protective coatings.
Performance
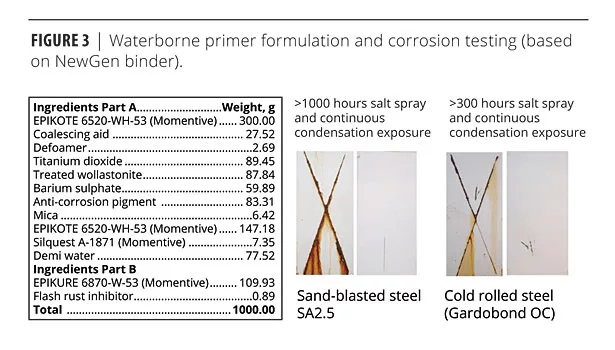
The corrosion resistance delivered by this system is illustrated in Figure 3. The coating was sprayed onto sand-blasted steel panels, cured for one week at room temperature and then exposed to salt spray and continuous condensation for 1000 h. After 300 h of salt spray and continuous condensation exposure, the NewGen formulation displayed excellent corrosion resistance as well as superior adhesion to cold rolled steel.
But what if we wanted even better corrosion resistance while still conforming to the new regulations? Adding zinc to solventborne primers enhances their corrosion resistance. Is it possible to make a waterborne 2K, low-VOC, corrosion-resistant epoxy primer system that outperforms commercial solventborne epoxy zinc-rich primers?
What Are the Challenges?
In aqueous formulations, zinc reacts with water, forming zinc hydroxide and releasing hydrogen (eq 1).
Zn + 2H2O àZn(OH)2 +H2 á↑ [1]
Because of this, the main challenge in creating a zinc-rich waterborne primer is to prevent the zinc from reacting with water during storage and application. Adding untreated zinc dust directly into aqueous components must be avoided to prevent the hazards associated with hydrogen generation.
Evolving Methodologies
Waterborne 3K systems are available, with the zinc being added as a third component to the aqueous binder just before paint application. This approach is complicated by the need for proper mixing of multiple components, as well as the potential health and safety issues regarding inhalation when incorporating powdered zinc. It is typically limited to the use of 2K coatings with 1 h application lives.
For easier application of waterborne zinc-rich epoxy coatings it is necessary to protect the zinc from the water, without deactivating it. The zinc metal must still be available as a sacrificial oxidant to protect the iron-containing substrates being coated.
One approach to this problem is described by Momentive Performance Materials.3,4 A zinc-rich paste was made by dispersing the zinc dust into a silane hydrolyzate, which protects the zinc and prevents it from reacting with water. Then the epoxy dispersion was added to this slurry. Another variation is to disperse the zinc dust into a pre-mix of the silane hydrolyzate and the epoxy resin dispersion.
The stability of these zinc pastes was tested by measuring for hydrogen outgassing at 40 °C. The 3K formulation failed after a few hours. The 2K silane hydrolyzates were significantly more stable at ambient temperature for up to 6 months. However, they exhibited some hydrogen outgassing when stored at 40 °C. Thus, more work was needed to develop a 2K zinc-rich primer that was stable even at elevated temperatures.
What if the zinc were added to the amine curing agent instead of the epoxy resin component? “Hybrid” waterborne epoxy systems have been developed in which zinc is dispersed in a high-solids, non-aqueous amine curing agent. This zinc-rich curing agent is then combined with a waterborne epoxy dispersion to form a 2K coating. Unfortunately, after the two components are mixed, hydrogen outgassing often begins quite strongly and the working pot life is not very long. Clearly, a new modified curing agent needed to be developed.
Preferred Approach
A new water-free amine − EPIKURE Curing Agent 8538-Y-68 − has been designed for use in waterborne zinc-rich primers. This amine curing agent works by quickly saturating the zinc surface with reactive hydrophobic polymers that encapsulate and protect the zinc from water. It performs well even in primers loaded with 80% zinc by weight. Once the amine components are reacted with the epoxy, the zinc surface again becomes available as a sacrificial electrolytic surface to form oxides that act as a corrosion barrier for iron-containing substrates. By using EPIKURE Curing Agent 8538-Y-68 in a highly filled zinc paste, with the appropriate waterborne epoxy dispersion, more than 4 h of working pot life is achieved with no outgassing.
The advantages of this new amine curative for use in epoxy zinc-rich primers are:
• Easier dispersion of the zinc powder;
• High-solids, acid-free and non-aqueous amine to minimize outgassing with zinc;
• Shelf stable: very limited to no detectable outgassing during zinc paste storage;
• Pot life: significantly longer pot life than other waterborne zinc alternatives;
• Suitable for use in non-zinc coating systems;
• Excellent intercoat adhesion with other waterborne mid- and topcoats;
• Excellent corrosion protection.
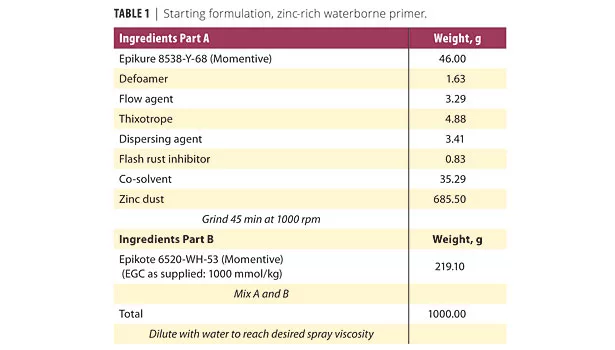
For best performance, this new amine curative should be combined with a stable dispersion of solid epoxy resin in water. The choice of epoxy dispersion resin is critical for obtaining long pot life, good paint coalescence and adequate crosslinking. Based on a survey of commercial dispersions, EPI-REZ Resin 6520-WH-53 was determined to be the best option. Care should also be taken to select non-acidic additives, particularly for the EPIKURE Curing Agent 8538-Y-68 zinc paste. An example of a formulation with superior performance is given in Table 1.
This formulation can be made to dry very quickly by selecting appropriate alcohol and glycol ether solvents. Typically this formulation has a pot life of at least 4 h. Recoat times can be minimized to only a few minutes if adequate air flow is available to accommodate water evaporation. There is no significant hydrogen outgassing during pot life, thus allowing a continuous coating barrier to be applied to protect the metal substrate.
As seen in Figure 4, salt spray-exposed steel panels coated with the formulation in Table 1 protect metal significantly better than the solventborne zinc-rich primer seen in Figure 5. This shows that the zinc is available for corrosion protection, even if significant outgassing is not observed during the pot life.
Conclusions
Zinc-rich 2K waterborne epoxy primers are now possible using new amine technology (EPIKURE Curing Agent 8538-Y-68). At the same zinc loading, the corrosion performance using this curing agent with solid epoxy dispersions is comparable to, or better than, that achieved with traditional solventborne epoxy/polyamidoamine binders.
The zinc-rich paste based on this new curing agent shows no significant outgassing when the curing agent and epoxy components are mixed. Resulting pot life with the recommended solid epoxy dispersion is at least 4 h.
The superior metal protection of NewGen-type, waterborne, 2K epoxy binder systems has been extended via a new curing agent technology that is suitable for highly filled zinc-rich primer formulations. This route allows significant VOC reduction in the important application area of organic zinc-rich primers.
References
1 Heine, F., et al. Waterborne Epoxy Technologies, EuRAILmag, Oct., 2007.
2Elmore, J., et al. WB Epoxy Protective Coatings for Metal, JCT, Vol 74, No 931, Aug., 2002.
3Heine, F., et al. Waterborne epoxy coatings in the marine industry: an option?, Nürnberg Coating Show 2009.
4 Lejeune, A., et al. New class of epoxy functional silanes for uses in coatings, adhesives and sealants: epoxy silane oligomers, 21st National OCCA Symposium in conjunction with SAPMA, Oct., 2006.n
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!





