The binders used in water-based paints are traditionally produced by emulsion polymerization. These binders are typically acrylic, styrene-acrylic or vinyl acetate based oil-in-water emulsion (dispersion) polymers.
One important property of polymer binders is their pigment binding capacity (PBC). The PBC of a polymer binder is reflected in the abrasion resistance the binder imparts to a paint film. This property can be tested with a standard wet scrub resistance test.1 The use of binders with high PBC allows for the formulation of water-based paints at relatively high pigment volume concentration (PVC) i.e., 45-55 weight percent (wt%) of the total formulation mass, with an 8-10 wt% binder composition. The use of such binders gives a cost reduction in the resultant paint, as binders are generally more expensive than extenders (calcium carbonate, talc).2 The PBC of emulsion binders can be adjusted by the type and amount of the auxiliary monomer (e.g., methacrylic acid (MAA) or acrylic acid (AA)).3 The role of the auxiliary monomer in an emulsion system is twofold: (a) it enables latex stability and (b) facilitates pigment wetting/binding.4
Cowles et al. showed that an acrylic polymer, termed the hydrosol, synthesized by solution polymerization, could also be used as a paint binder.5 The hydrosol backbone is composed of styrene and typical acrylate monomers such as n-butyl acrylate used in the synthesis of water-based binders. These two main monomers are copolymerized with either MAA or AA. A base, such as ammonia, is then added to the hydrosol to neutralize the carboxylic groups within the polymer backbone. The neutralized groups render the hydrosol miscible with water to be used as a binder in water-based paints. After application of the hydrosol paint onto the desired surface, evaporation of the volatile ammonia returns the polymer to its original hydrophobic nature. A benefit is seen in the adhesion of the solution-based polymer to inorganic particles when compared to the emulsion binders. We hypothesize that the improved performance of the hydrosol is due to the absence of surfactants, which are required in emulsions for stability, as well as the increase in the carboxylic functionality.
Binding Power of the Hydrosol Polymer
The abrasive performance of a coating is an important property, especially for interior application of water-based coatings. Abrasion resistance is influenced by the size, type and concentration of the pigments used in the formulation of the coating, as well as by the hardness and type of binder.
It is known that a decrease in the pigment concentration increases the abrasive performance of the coating but at the same time other key properties of the coating, such as opacity and color, will be affected. Decreasing the pigment relative to the binder concentration will also increase the cost, as most extenders are less costly than the binders.
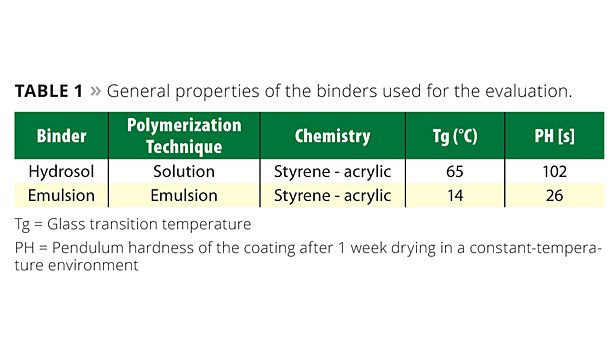
It is therefore reasonable that research be undertaken whereby abrasive-resistant binders are developed. One such type of binder is the hydrosol. Due to a large amount of carboxylic groups present in hydrosols, a strong association between the hydrosol and the pigment surface exists, effectively increasing the PBC. The higher concentration of carboxylic groups increases the modulus of the hydrosol polymer. When compared to a standard emulsion binder, the hydrosol polymer is considerably harder. The increase in hardness is related to the higher glass transition temperature (Tg) of the hydrosol polymer. The reason is that the functional carboxylic monomers dramatically increase the polymer Tg according to the Fox equation.6 This phenomenon is clearly seen when comparing the König pendulum hardness measurements of the hydrosol polymer to a standard emulsion binder at a constant film thickness. Basic properties of the two binders can be found in Table 1.
Binder hardness has a direct influence on the minimum film forming temperature (MFFT) of the final coating. Even though large differences between the hardness results for the emulsion binder (22-27 seconds) and the hydrosol binder (100-105 seconds) were seen, the final optimized paint formulation formed a film at room temperature. A major benefit of the hydrosol polymer over traditional emulsion binders, therefore, is that it can be formulated to produce relatively hard, high-Tg films while film formation can still occur at room temperature without the need for additional coalescent/co-solvents in the final coating.
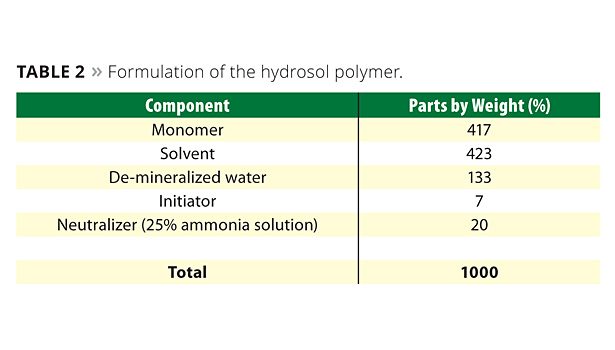
The manufacture of the hydrosol requires a suitable solvent for the polymerization of the monomers. A typical formulation for the hydrosol polymer is shown in Table 2. The solvent used should be low in toxicity but also present sufficiently low chain transfer during polymerization to allow the polymer to reach the desired molecular weight. Typical solvents are diacetone alcohol and isopropanol. The solvent remains in the hydrosol binder and effectively acts as coalescing solvent in the paint formulation, replacing the traditional coalescing solvents used in emulsion binder paints.
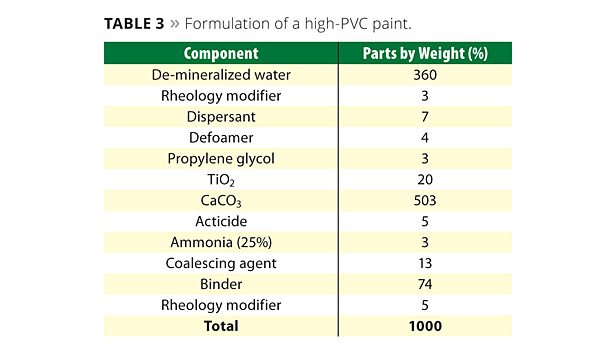
A second key property of the hydrosol that contributes to the final coating integrity is the relatively strong association to the pigment surface when compared to emulsion binders. Due to this strong association to the pigment surface, the binding power of the polymer is increased.7 Experimental work showed improved scrub resistance of the hydrosol-based coatings when compared to the same coating formulation using a standard emulsion binder (at equivalent polymer solids). An example of a high-PVC coating formulation used to evaluate the above test is shown in Table 3.
Reduction in Binder Content
An investigation was conducted to evaluate the usage of the hydrosol at varying levels in a high-PVC coating. The aim was to assess if the hydrosol could be used at a lower level than the standard emulsion binder, as the high Tg and strong association to the pigment surface should result in a higher binding power. In this investigation various coatings were prepared based on the composition in Table 3.
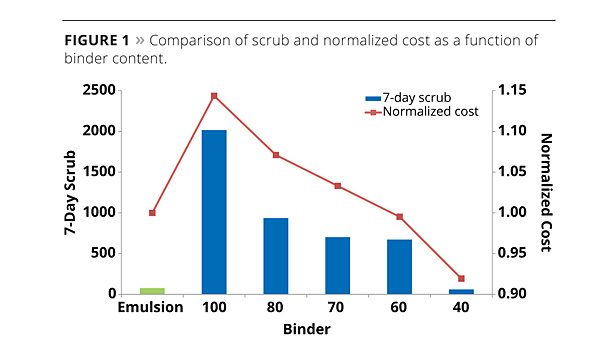
The hydrosol was added to the coating formulation at equivalent polymer solids to the standard binder and reduced incrementally until comparable scrubs were achieved as that of the standard. Scrub panels were analyzed for abrasive performance 7 days after the drawdown was prepared. The normalized costs for the final coatings were compared to assess a possible cost saving that could be achieved by decreasing the binder load. The results obtained are represented graphically in Figure 1. Along the x-axis of Figure 1, the hydrosol binders are denoted as a percentage of polymer solids relative to the standard binder. The standard binder used for the evaluation is the emulsion polymer characterized in Table 1.
The 7-day scrub results are shown on the primary axis of Figure 1. Replacing the emulsion binder 1:1 with the hydrosol binder shows a dramatic increase in the film strength with the scrub resistance increasing from 75 scrubs for the standard coating to beyond 2000 scrubs for the hydrosol coating. However, a significant increase in the overall cost of the final paint was seen. As the level of hydrosol was decreased, the scrub resistance of the coating decreased and the cost could also be reduced. At 40 wt% equivalent polymer solids to the standard, comparable scrub resistance was achieved at a lower overall cost of the final paint. The decrease in abrasion performance can be attributed to the increase in the ratio between pigment and binder. The lower binder content results in a coating with an increased pigment/extender composition; this greatly affects the durability of the coating. With a decrease in binder content a subsequent decrease in abrasion performance is expected, as the binding power of the coating has been reduced.
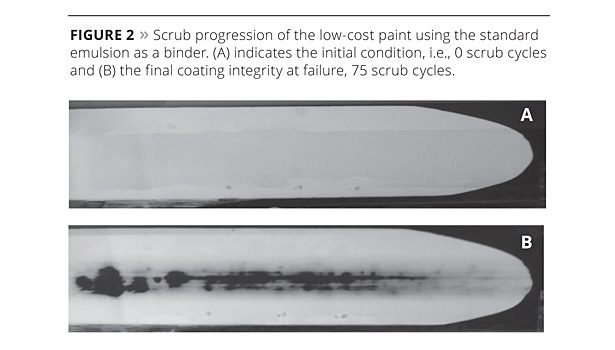
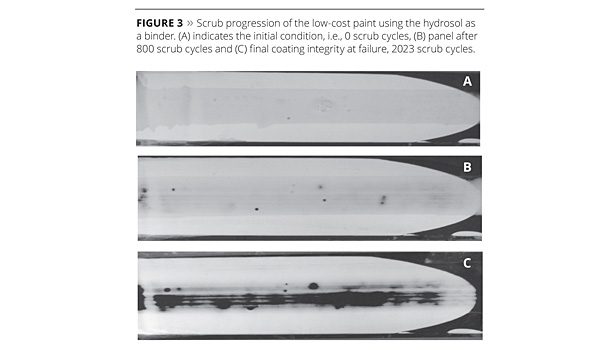
The abrasion test results for the emulsion binder and the 1:1 replacement of the emulsion binder with the hydrosol binder are shown in Figures 2 and 3, respectively. Figure 2 (A) shows the high-PVC coating of the emulsion binder prior to testing, and Figure 2 (B) at failure after 75 scrubs. Figure 3 (A) shows the initial state of the hydrosol binder compared to Figure 3 (B) at 800 cycles and Figure 3 (C) at 2023 cycles. Visually it is clear that for the same level of polymer added, the hydrosol performance is superior in terms of scrub resistance. The large improvement in the scrub resistance is critical for high-PVC paints, as high pigment content generally leads to poorer abrasion resistance when compared to coatings with higher binder content.
The Hydrosol Polymer as a Dispersant
Dispersants prevent agglomeration of the pigment particles and, therefore, form an integral part of any paint formulation. In general, polyacrylate dispersants provide stable dispersions of pigment particles in the millbase by associating with the electrically charged surface of the pigment or extender. It is well known that pigment particle size and the stable dispersion thereof greatly affect the optical properties and flow behavior of the coating.
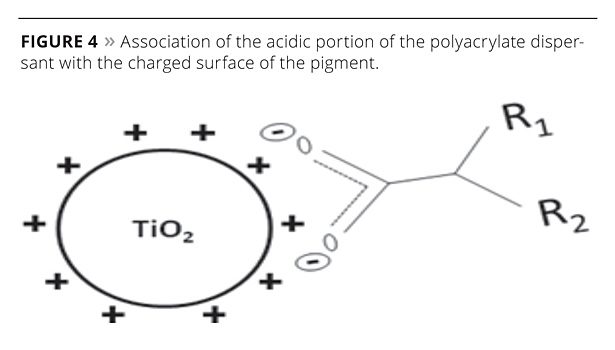
In the case of the titanium dioxide pigment, the surface of the pigment particle is positively charged below a critical pH value, i.e., the isoelectric point. Polyacrylate dispersants, having a high acidic content, are electrostatically attracted to the positive pigment surface, as the acid functionality within the polyacrylate chain is negatively charged, as shown in Figure 4.
The electrostatic attraction between pigment and polymer allows a stable dispersion to be maintained. The polymer provides a buffer layer between the separate primary pigment particles via a combination of electrostatic repulsion and steric hindrance.8
It was expected that the high concentration of carboxylic groups present in the hydrosol binder could improve pigment dispersion. Even though the primary function of the hydrosol polymer is to serve as a binder, it is imaginable that the hydrosol could have a dual functionality and also function as a dispersant. The benefit is twofold in that firstly the formulation of the coating may be simplified by removing other dispersants, and secondly a price reduction in the coating may be afforded by the removal of other dispersants.
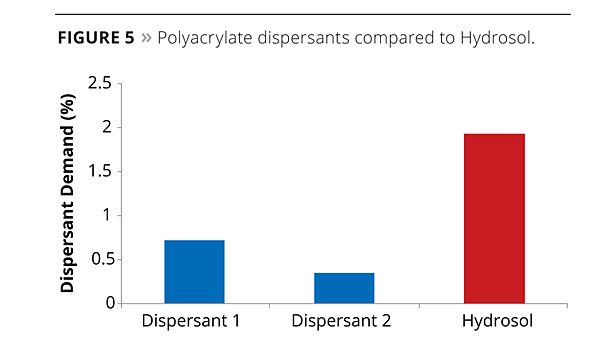
To assess the effectiveness of the hydrosol as a dispersant, two known polyacrylate dispersants were compared to the hydrosol using a dispersant demand test. The test method involved the production of a millbase without the addition of any rheology modifiers. The dispersant being evaluated was then added incrementally to the millbase to determine the minimum amount of dispersant required to obtain a constant viscosity.
The dispersant demand for two commonly used dispersants compared to the hydrosol polymer is shown in Figure 5. The minimum amount of the hydrosol polymer required to produce a constant viscosity was found to be 2% of the total formulation mass; this is well within the typically added level of 7-10 wt% binder composition in a high-PVC coating. The key result was that the hydrosol, due to its particular chemical composition, could function both as a dispersant for the pigments in the millbase preparation stage and as the binder in the letdown stage. In this way the hydrosol could be added at a particular level to obtain a reasonable millbase viscosity and subsequently increased during the letdown stage to the desired binder concentration.
Conclusions
Due to environmental concerns and legislation, aqueous-based coatings are becoming the dominant binder choice over solvent-based coatings where cost and performance can be matched. For this reason, innovative binders need to be developed that show comparable or improved performance.
In this investigation a solution polymer was synthesized that is miscible in water and could be formulated to an aqueous-based coating without the use of surfactants or dispersants. The absence of surfactants in the solution polymer prevents possible surfactant leaching in the dried final coating and the negative properties associated with it. The polymer, termed the hydrosol, showed excellent abrasion resistance when compared to a standard emulsion binder. It was shown that a decrease in polymer solids by more than 50 wt% when using the hydrosol could still produce comparable scrubs to the standard coating without increasing cost. When the hydrosol was used at a level that produced an equivalent coating cost, the abrasive resistance of the coating was increased nine times compared to that of the standard.
To conclude, the hydrosol has shown promise in various ways, including (i) removal of surfactants from the binder formulation, (ii) abrasive performance enhancement, (iii) cost savings and (iv) process simplification of the final coating. The hydrosol has, therefore, shown promise as a suitable binder for the next generation of water-based paints. n
References
1 Kirsch, S.; Pfau, A.; Frechen, T.; Schrof, W.; Pföhler, P.; Francke, D. Progress in Organic Coatings2001, 43, 99.
2 Smit, A.; Dersch, R; et al. Flat Paint Based on Advanced Binder Technology; 2000. (BASF Corporation and BASF AG).
3 Warson, H.; Finch, C. A. Applications of Synthetic Resin Latices; Wiley: New York, 2001; Vol. 2: Latices in Surface Coatings Emulsion Paints, p 703.
4 Zosel, A.; Heckmonn, W.; Ley, G.; Machtle, W. Colloid Polymer Science1987, 265, 113.
5 Cowles, R.A; Georgalas, N; Shah, S. C. BASF Corporation, United States Patent, Sep 10, 1991.
6 Fox, T.J.; Loshaek, S.J. Influence of molecular weight and degree of crosslinking on the specific volume and glass temperature of polymers. Journal of Polymer Science1955, 371: 1.
7 Beatty, L.W.; Penboss, I. Film Formation, Suface Coatings: Raw materials and their usage, The New South Wales University Press, 1993, Vol 1, pp 327-329.
8 Kirish, S.; Stubbs, J.; Leuninger, J.; Pfau, A.; Sundberg, D. Journal of Applied Polymer Science, 2004, 91, 2610.
9 Creutz, S; Jerome, R; Kaptijn, G M P; A W van der Werf; Akkerman, J.M. Design of polymeric dispersants for waterborne coatings. Journal of Coatings Technology 1998, 70, 883, 41.
Acknowledgments
The authors wish to thank the management of Plascon SA for granting publication of this manuscript and the analytical team at Kansai Plascon Research Centre for their support.





Report Abusive Comment