Conductive Polymer Nanodispersion
Anti-Corrosion Coating Technology


Current coating technology utilizes heavy-metal anti-corrosion pigments in the primer to provide high-performance/heavy-duty corrosion protections for metals. For years, the most effective anti-corrosion pigments were those that contained hexavalent chromium (Cr6+), which is highly toxic and carcinogenic. The toxicity of Cr6+ became well known to the general public through the 2000 Oscar-nominated film Erin Brockovich. The film depicted the real-life story of hexavalent chromium leakage that contaminated both groundwater and drinking water in the small California desert town of Hinkley, and led to the largest legal settlement in U.S. history.
Hexavalent chromium is a known carcinogen for humans and it may contaminate water, soil and air, and harm plants and animals alike. A 2010 study by the Environmental Working Group (EWG) discovered that Cr6+ was present in the tap water of 31 of the 35 U.S. cities sampled, and 25 of them were at a level above the California-proposed safe maximum.1
Hexavalent chromium was named the number-one toxic enemy of the U.S. military.2 Without an effective alternative, Cr6+ is still the choice for high-end corrosion protection such as the military and aerospace industries for protecting their mission-critical assets.
Chromic acids are among the first chemicals to require authorization according to European REACH (Registration, Evaluation, Authorization, and Restriction of Chemical substances) rules, and have been set for banning/restricted usage in 2016. The European RoHS (Restriction of Hazardous Substances Directive) law has restricted Cr6+ on most electronics. New EPA regulations were proposed for early 2012 to provide guidance for decreasing the allowable Cr6+ emissions from plating, anodizing, chromate conversion coating, and sealing processes.3 OSHA’s new rules also require minimizing Cr6+ exposure in the work place. These tightened regulations will result in product deselection and market losses without an effective alternative.
Zinc and/or zinc compounds are current environmentally compliant, industry-standard chromium alternatives. However, zinc cannot protect aluminum (a major material for the aerospace industry) or galvanized steel, and is less effective in severely corrosive environments. The price of zinc is expected to increase4 and, according to figures from the United States Geological Survey, with current usage levels, the known zinc reserve will be depleted around 2020.4 Over 50% of zinc has been used to protect metals from corrosion either through the galvanization process or as the sacrificial anti-corrosion pigment in coatings. Industries have long been diligently searching for an effective Cr6+ alternative pigment for corrosion protection.
Development of Polyaniline (PAn) Coatings
Conductive polymers have been regarded as an environmentally friendly alternative to hexavalent chromium pigments since the discovery of the polyaniline (PAn) anti-corrosion effect in the early1980s.5 That PAn can be used as a corrosion inhibitor was also stated in the 2000 Nobel Chemistry award publication.6 Many companies, institutions and universities have invested significant resources into this research, and hundreds of papers and patents have been published. Although research has shown some positive results, the development of commercial products has been a long and arduous path over the past 30-plus years. In the 1980s, Allied-Signal Co. successfully produced a PAn product that is dissolved in 1-methyl-2-pyrrolidone (NMP) with low concentration (~ 5%), however it is very difficult to disperse in the organic resins and solvents used in paint formulations. In the 1990s, Monsanto Co. had a breakthrough invention of soluble PAn, which is a fine dispersion with a stabilization layer adsorbed on the particle surface.7 Paints formulated with this product passed several hundred hours of salt fog tests.8 Around the year 2000, Ormecon Co. developed an organic metal PAn product,7 and the related anti-corrosion paint successfully passed 1,035 h of salt fog testing (DIN SS 50 021).8 Without adhesion test results, this product failed in the market due to weak adhesion and also high material cost. There is still no conductive polymer anti-corrosion product on the market today.
CPND Anti-Corrosion Coatings
To meet the demand for an environmentally friendly yet high-performance anti-corrosive, AnCatt Co. has successfully developed conductive polymer nanodispersions (CPNDs). Conductive polymers, also known as inherently conductive polymers (ICPs), were a Nobel Chemistry winning material in 2000: transparent, flexible and conductive, which has long been expected to usher in a new era in materials science. However the processing difficulties prevented many conductive polymer applications from becoming a reality. CPNDs are soluble in many organic solvents, such as ethanol, and are extrudable. The unique nanoparticles are in consistent spindle shapes, approximately 100 nm long and 50 nm wide (Figure 1). CPNDs should enable large-scale commercialization of the conductive polymer applications that have long been anticipated, such as anti-corrosion primers, electrostatic dissipation coatings, electromagnetic interference shielding, static-resistant fibers, conductive inks, conductive toners, conductive adhesives, conductive textiles, etc.
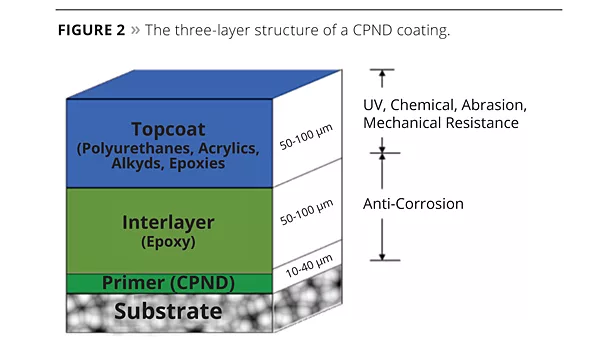
At present we use the nanoparticles as anti-corrosion pigments. The CPND coatings are made of three layers (Figure 2): the primer contains PAn nanoparticles, the interlayer is an epoxy layer, and the topcoat is composed of polyurethanes, acrylics, alkyds or epoxies. The primer layer is about 10-40 microns thick; the inter-layer and topcoat are each about 50-100 microns in thickness. The first two layers together provide excellent corrosion protection, and the third layer provides UV, chemical and abrasion resistance.
Salt Fog and Cyclic Weathering Exposures
AnCatt corrosion tests and the final report were provided by KTA, the most well-known third party independent corrosion test laboratory in the United States. Following a specified exposure time, specimens were removed, immediately rinsed with de-ionized water, and evaluated for visible changes in the appearance of the coatings. The panels were rated for blistering in accordance with ASTM D7149 and rusting in accordance with ASTM D610.10 For each method, the rating scale ranges from 0 to 10, with a rating of 10 indicating no blistering or rusting. Lower rating numbers represent progressively larger blisters and rusted areas.
At the ASTM standard maximum of 5,000 h of salt fog testing, the coatings still remained intact (Figure 3). Due to our own curiosity we continued to over 13,000 h of testing, and surprisingly both rusting and blistering ratings remained at the best possible score of 10. The back sides of the panels were coated with a standard acrylic coating. Figure 3 presents the pictures of the CPND coating vs. the acrylic coating on the backside at different salt fog exposure hours. The acrylic coating had light rusting at 1,000 h of exposure, but rust spots were spread out to the entire panel by 5,000 h. At 8,370 h of exposure, the acrylic coating was completely destroyed by rust and large blisters, even corroding through to the scribe line on the front. On the other side the CPND coating was still in good condition with excellent adhesion (the three “x” marks were the result of tape adhesion tests, and the three circle marks were the result of pull-off adhesion tests). The panels were visually rated for the amount of undercutting extending from the scribe line. Two panels were scraped at 8,372 h and the measured undercutting was avg. at 2 mm and 3 mm, respectively. They were then put back in the salt fog chambers for additional exposure until 12,740 h had elapsed. The additional exposure of 4,368 h did not increase the width of undercutting, which indicates that the scribe marks had sealed themselves from further rusting. The salt fog performance of the CPND coating predicts a much extended performance life as a protective coating, thereby prolonging the life of the metal structures they protect, lowering metal structure depreciation rate, and realizing reductions in waste metal, metal structure maintenance/costs, and indirect costs related to coating failures. For more detailed test data and additional test data on cyclic weathering, aluminum substrates, please see the full KTA lab report at www.ancatt.com.
Adhesion Test Results
Adhesion between the metal, primer and topcoat is crucial for the success of an anti-corrosion coating system. The adhesion of the CPND coating was tested after accelerated corrosion exposure. Pull-off adhesion test scores ranged from 650 psi to 850 psi after 1,100 h of cyclic weathering testing. After 8,372 h of salt fog testing, pull-off adhesion test results were still in the very good range of 560 psi. These results indicated very good adhesion according to SSPC Good Painting Practice (above 200 psi is considered good coating adhesion).11 The tape adhesion test received the best possible score of 5A for each of the three replicates after 8,372 h of salt fog exposure.
Electrochemical Measurement Results
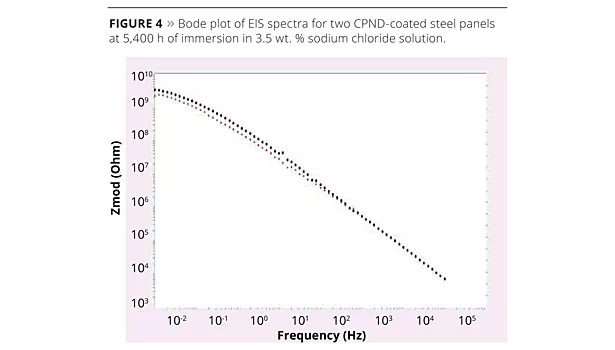
Electrochemical impedance spectroscopy (EIS) studies the pore resistance to electrolyte penetration through the coating layer. For a Bode plot of EIS spectra, when frequency (Hz) approaches zero, the modulus of impedance is taken as the pore resistance (Ohm). The resistance value may decrease during the exposure period; the resistance of a poor coating will continue to decrease, resulting in failure of the coating. A prior study showed that a coating that maintained a resistance of 108 Ohm cm2 provided good corrosion protection, while one whose resistance fell below 106 Ohm cm2 did not.12 Two CPND-coated steel panels were immersed in 3.5 wt% sodium chloride solutions for over 5,400 h. The pore resistances were over 109 Ohm (Figure 4). Since the exposed coating surface area is 3.5 cm2, the pore resistances are over 3.5 x 109 Ohm cm2.
Many researchers have found that, when compared to a bare steel sample, a PAn coating makes the corrosion potential more noble. The Tafel plots of the two CPND-coated panels (Figure 5) show about + 700 mV shifting after over 5,400 h of immersion.
Conclusions
Independent corrosion tests have shown this is a successful conductive polymer-based anti-corrosion coating technology and the first non-chromate coating that has out-performed hexavalent chromate coatings. It is also the first heavy-metal-free heavy-duty anti-corrosion coating technology. It shows impressive results when compared to current coating performances. The CPND pigments and the unique coating formulation are readily manufactured in scalable processes at a cost very competitive with other anti-corrosion systems.
The CPND anti-corrosion coating platform can protect a wide range of metals under severe corrosive environments. CPND coatings could be further applied to anti-corrosion coating applications such as high temperature, high solids, powder and waterborne systems. CPND coating technology will result in:
- tremendous economic benefits by significantly elongating the performance life of anti-corrosion coatings and, therefore, the performance life of the metal structures they protect;
- the reduction of waste metals, coating maintenance and repaint costs, as well as corrosion-related accidents, injuries, fire and delays;
- helping create a greener planet and a brighter future by preventing toxic heavy metals from polluting air, soil and water systems.
- preserving the zinc reserves on the earth, and solving the zinc shortage we face in the near future.
The AnCatt CPND coating brings protective coating technology to new heights of sustainability and performance. The high-loading CPND approach shows promise not only for corrosion resistance, but also as a technology readily incorporated into a wide variety of coating types, including automotive, aerospace, marine and industrial finishes.
Author’s Note:
CPND technology was the winner of the 2012 American Chemical Society Green Chemistry and Engineering Business Plan Competition, the 2013 University of Delaware Business Plan Competition, and the 2013 National Innovation Award.
References
1 Layton, L. Probable carcinogen hexavalent chromium found in drinking water of 31 U.S. cities, Washington Post, Dec. 19, 2010, http://www.washingtonpost.com/wpdyn/content/article/2010/12/18/AR2010121802810.html.
2 Casey, T. U.S. Military Targets Toxic Enemy #1: Hexavalent Chromium, Clean Technica, 2009, http://cleantechnica.com/2009/07/04/us-military-targets-toxic-enemy-1-hexavalent-chromium/.
3 ASETS Defense 2012: Sustainable Surface Engineering for Aerospace and Defense, Aug. 27-30, 2012, http://workshop.asetsdefense.org/.
4 Helias A. Scarcity of micronutrients in soil, feed, food, and mineral reserves, Woortman, Natural Resources, Sept. 4, 2012, http://www.iatp.org/documents/scarcity-of-micronutrients-in-soil-feed-food-and-mineral-reserves (Oct. 31, 2012).
5 Beberry, D.W. J. Electrochem, Soc. 1985, 132, 1022.
6 Heager, A.; MacDiarmid, A.; Shirakawa, H. For the discovery and development of conductive polymers, Nobel Prize in Chemistry 2000, http://www.nobelprize.org/nobel_prizes/chemistry/laureates/2000/popular.html (Oct. 31, 2012).
7 Miller; Granville, G. et all. USP 5,853,621.
8 Wessling, B. JCSE volume 1, Paper 15.
9 Standard Test Method for Evaluating Degree of Blistering of Paints. ASTM International (ASTM), 100 Barr harbor Dr., West Conshohocken, PA 19428-2959.
10 Standard Test Method for Evaluating Degree of Rusting on Painted Steel Surfaces. ASTM International (ASTM), 100 Barr harbor Dr., West Conshohocken, PA 19428-2959.
11 Drinsko, R.W. Good Painting Practice, SSPC publication.
12 Greenfield, D.; Scantlebury, D. JCSE vol 3 Paper 5.
This article originally appeared in Coating Technology and Abstracts.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!






