Aprocess to synthesize hydroxyl-terminated polycarbonates from carbon dioxide and epoxies was originally developed a number of years ago.1 This is a fertile research area, and the reference is one of many issued patents in the field. The promise of using carbon dioxide as an inexpensive and renewable raw material to produce polymers was sufficiently attractive that Novomer, Inc. was founded to commercialize this technology. Building upon this pioneering work of Coates et al., Novomer has advanced the catalyst technology and developed a family of proprietary catalysts with improved activity and selectivity. These catalysts maximize carbon dioxide incorporation and provide polycarbonates with perfectly alternating CO2/epoxide monomer incorporation. These polymers also have low polydispersity indices. In addition to improving the performance of the resulting polymers, these catalysts allow Novomer to conduct polymerization using parts per million catalyst levels. Not only can the product be labeled “green”, but it also offers advantages as a commercial raw material that can be made using waste carbon dioxide.
Hardcoats to improve the surface properties of plastics, glass and metals are commercially available. This work examines hardcoats that can be applied to plastic and glass. They could be applied to metals, but the work has not progressed in that direction yet. Commercial hardcoats for plastic and glass generally are very low-solids formulations. They may also be cured by UV radiation.
The use of Novomer polycarbonate polyols to make hardcoats started when other work with these materials showed their inherent adhesive and solvent-resistant properties. The hardcoats described in this work are made by adding an isocyanate A side to a B side containing a polycarbonate diol. The hardcoat formulations may be catalyzed, as well as have other additives incorporated into them. Even though these formulations are a two-part system, their pot life is long enough that they may be treated as a one-part curable system.
Experimental
Adhesion testing was performed according to ASTM D3359 using a six blade cutter. Typically, the cutter was used to make a long cut. Then the cutter was used to make multiple, transverse cuts at 90° to the long cut. The scribed cuts were brushed with a soft brush and adhesive tape firmly applied to the scored areas. The tape was removed and adhesion measured by comparison to the ASTM/ISO adhesion scale. A rating of 5B is best.
Pencil hardness was performed according to ASTM D3363. A Gardco pencil holder was used. The pencil hardness was tested until a non-removable mark was formed on the hardcoat. Pencil hardness was recorded as the pencil used previously to the one making the non-removable mark.
Resistance to UV radiation was tested by exposure to UVB radiation. Coated samples were placed facing the UVB light source.
Solvent resistance was tested by immersing coated samples in various solvents, acids and a base. The properties of the coating were tested before and after exposure.
Results and Discussion
Hardcoats are made by combining an isocyanate-containing A side with a B side containing a hydroxyl-terminated polycarbonate resin. The B side contains solvents, and the solvent content ranges between 20 and 45 percent by weight, depending on the application and product requirements of the hardcoat.
High-boiling solvents have been successfully used to make hardcoats. Three examples of these solvents are PMA (propylene glycol methyl ether acetate), DBE (aliphatic dibasic esters) and DPMA (dipropylene glycol methyl acetate).
Hardcoats have been successfully applied by dip coating, spraying and brushing. The pot life can be several days, which allows the formulation to be used as a one-component mixture. The viscosity of the combined A and B sides is relatively low at 50 cps. The coated material sets in 90 min at room temperature, and is dry in about 220 min. Set and dry times can be accelerated by raising the temperature or the catalyst level.
Adhesion and Hardness
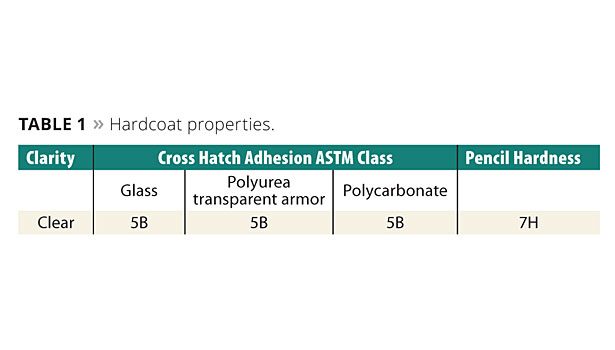
Typical hardcoat adhesion to several substrates and hardness values are shown in Table 1. The 5B level of adhesion is the highest measurable value using ASTM D3359. These hardcoats show good adhesion to a variety of substrates. The 7H pencil hardness is a reasonable level of hardness.
Properties may be modified by the use of additives commonly supplied to the coatings industry. Many of the additives improve performance while maintaining the clarity of the hardcoat. Properties can be improved by the choice of additive, but clarity is not always maintained with every additive.
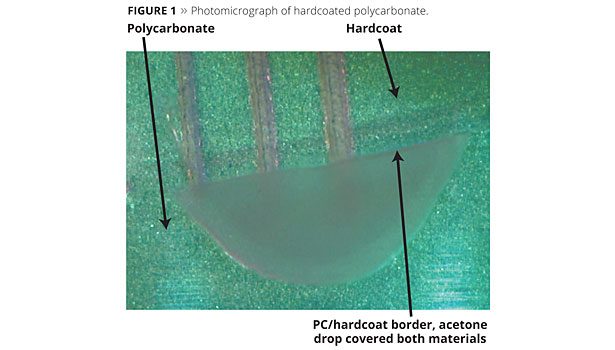
A photomicrograph of hardcoated polycarbonate is shown in Figure 1. The dark, etched lines are 2 mm apart. Uncoated polycarbonate at the bottom of the photo shows scratches made by rubbing steel wool across the surface of the polycarbonate. Scratches are not seen on the hardcoat treated in a similar manner.
A drop of acetone was placed at the boundary between uncoated and coated polycarbonate and allowed to dry. The uncoated polycarbonate turned cloudy in the area that was in contact with acetone. The hardcoat remained clear where it was treated with acetone.
UV Stability
Hardcoats on glass were exposed to UVB radiation continuously for six months in a QUV test chamber. Neither the adhesion to glass nor the pencil hardness was affected by this exposure. In addition, the coating neither cracked nor yellowed. Glass was used as the substrate since it would not yellow or crack under these conditions and complicate data analysis. The results are promising and long-term tests are being done.
Solvent Resistance
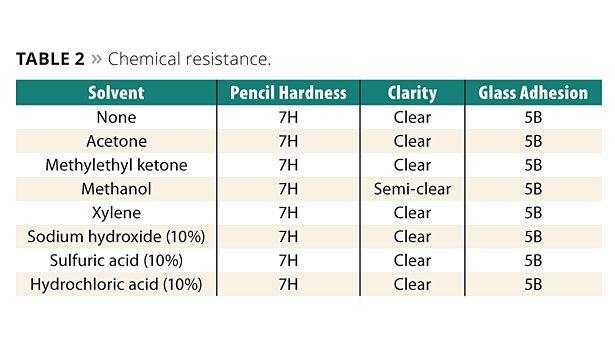
Solvent resistance results for hardcoats are shown in Table 2. Twenty-four-hour immersion test results show that the hardcoats are not impacted except for methanol, which causes the hardcoat to become semi-clear rather than clear. The hardcoat is not affected. Immersion tests were performed by coating the hardcoat on glass because it is not affected by the solvents, acids and base as would the plastic substrates.
Abrasion Resistance
Abrasion resistance testing was performed using polycarbonate as a control. The hardcoat lost only one-half of the material abraded from polycarbonate in a Taber abrasion test. The abrasion resistance was also examined qualitatively by rubbing the hardcoat and polycarbonate with steel wool. The hardcoat remained unaffected, while the polycarbonate showed visible scratching.
Temperature Cycling
Glass coated with the hardcoat was tested after exposure to ambient conditions, 24 h at -20 °C and 24 h at 60 °C.The pencil hardness, adhesion and clarity were not affected by this temperature exposure.
Summary
The carbon dioxide-based hardcoats developed in this project offer a unique balance of properties that make them viable alternatives to existing hardcoat technologies, in particular for substances such as thermoplastic polycarbonate. From a formulating perspective, the system is quite flexible. It can be cured at elevated temperatures and offers a long pot life, enabling use as either a one- or two-component system. Its low viscosity allows for multiple application techniques including dip coating, spraying and brushing.
The finished hardcoat demonstrates an attractive balance of hardness, adhesion, chemical/solvent resistance, temperature resistance and abrasion resistance. The formulations tested here yielded pencil hardness of 7H, crosshatch adhesion to thermoplastic polycarbonate of 5B, improved abrasion resistance vs. untreated polycarbonate, excellent UV aging, and good resistance to numerous acids and bases. The Hanson Group and Novomer are currently commercializing high-performance transparent hardcoats similar to the system described herein with a particular focus on thermoplastic polycarbonate and polyurea transparent armor substrates.
References
1 Coates, G.W.; Chen, M. 2000. Polycarbonates made Using High Activity Catalysts, US Patent 6,133,402.
This paper was presented at the 2013 Polyurethanes Technical Conference, Sept. 23-25, Phoenix, AZ.




Report Abusive Comment