
Currently, Europe consumes more than 350,000 metric tons of powder coating per year. The annual rate worldwide is 1,000,000 metric tons - and rising (Figure 1). By 2008, industry experts anticipate global demand to reach over 1.2 million metric tons, of which Europe will account for 500,000 metric tons.
Polyurethane (PU) coatings are known for their excellent coating properties, good weather stability and versatility of formulation. In particular, powder coating technology - besides water-based dispersions - has made it possible to apply PU formulations in a solvent-free and, hence, environmentally friendly manner. However, conventional PU powder coatings release blocking agents during the hardening process, which are undesired both ecologically and economically.2 Internally blocked and, hence, emission-free PU powder coatings do not have this drawback.3 Unfortunately, up to now these blocking agent-free PU powder coatings required hardening temperatures of 180 °C and higher, excluding the possibility of applying these coatings on temperature-sensible substrates like wood, plastics and pre-assembled parts. In this article novel catalysts are described, which allow decreasing the hardening temperature to 120 °C and even lower.
Experimental
a. 13C-NMR-Spectroscopy: Bruker AMX 500 (500 MHz), Internal Standard TMS (optionally, relaxant chromacetyl acetonate); 13C-NMR spectra were 1H-broad band-decoupled.b. Starting material: Isophorone diisocyanate (IPDI) uretdione were made at Degussa AG, Coatings & Colorants. Ethylhexanol and catalysts were purchased from Aldrich. The uretdione content was measured by reaction with butyl amine followed by back titration of excess amine with hydrochloric acid.
c. Powder coatings: Crosslinker: VESTAGON EP BF 9030, Degussa AG, latent NCO-content 12.2 %, Tg 41- 44 °C; polyester resin: Crylcoat 2839, Cytec, OH-value 58 mg KOH/g, Tg 57 °C; pigment: titanium dioxide, KRONOS 2160, Kronos; acid scavenger: ARALDITE PT 912, Vantico; auxiliary agents: benzoin (degassing agent, Aldrich), RESIFLOW PV88 (levelling agent, Worlee.)
Uretdione
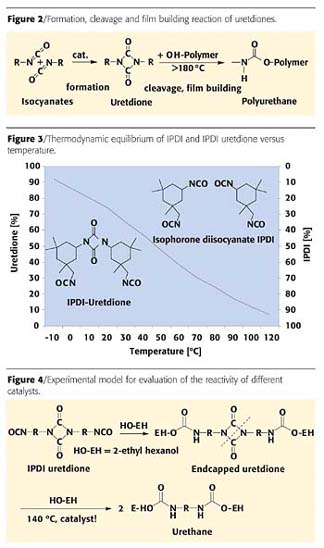
Uretdione denotes a four-membered heterocyclic compound obtained by suitable catalysis from dimerization of two isocyanate groups (Figure 2). Such compounds are thermally labile; that is, they split into their original components under the influence of temperature. The isocyanate groups released can then react with a hydroxyl group-containing resin to harden the coating film.3 For such uretdiones, isophorone diisocyanate (IPDI) is now used almost exclusively as a starting material.If we look at the thermodynamic equilibrium of the uretdione formation of IPDI we can observe that at low temperatures (around room temperature) the dimer is favored, whereas at 120 °C the monomer dominates (Figure 3). Thus, it is surprising that conventional curing of uretdione-based formulations takes place at 180 °C and higher. The reason for this discrepancy lies in a kinetic hindrance of the cleavage reaction. To overcome this obstacle, suitable catalysts have been searched for over the past 20 years and found only recently.
Although IPDI uretdione is a mixture of 10 different isomers,4 which vary in selectivity,5 reactivity and concentration, in this study we focused just on an average composition.
Catalyst Evaluations
To test a huge amount of catalysts it was mandatory to find an easy way of evaluation, since a real powder coating includes extrusion, grinding, sieving, applying and curing of a formulation, consuming a lot of time. Thus we investigated a model for fast throughput testing of catalysts.IPDI-uretdione was blocked irreversibly with 2-ethyl hexanol at the outer NCO-groups, leaving the uretdione ring intact (Figure 4). In a second step, the reaction of the excess 2-ethyl hexanol with the isocyanate units of the uretdione ring at 140 °C in the time curve then enabled the reactivity of selected catalysts to be determined. The content of the remaining uretdione groups was used as a measure of conversion.
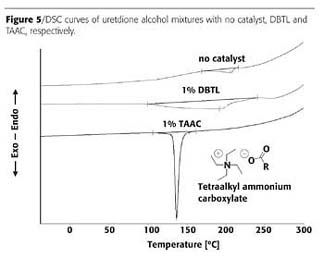
In addition, differential scanning calorimetry (DSC) was used as a hint for reactivity. In this way about 200 different catalysts were tested, and surprisingly, at least four different classes of useful catalysts were found. In Figure 5, the DSC curves can be seen for the uncatalyzed system, for dibutyltin dilaureate (DBTL) and for tetraalkyl ammonium carboxylate (TAAC) catalyzed formulations, respectively.
The activity of TAAC is strongly inhibited by acidic components, e.g., the remaining acid groups in OH-functional polyesters. In this case, compounds - so called acid scavengers - have to be added to intercept the acid groups and eventually to restore the activity of the catalyst. These acid scavengers are beneficial to adjust reactivity and in addition to improve the flow of the powder coating.
13C-NMR analysis revealed that besides the mainly formed urethane, isocyanurate groups are generated. The quantity of these structures depends on parameters like temperature, catalyst concentration and NCO/OH ratio. In the first few minutes of reaction even small amounts of allophanates could be detected, although after 30 minutes at 140 °C no allophanates are left, only urethanes (80%) and isocyanurates (20%). These side reactions lead to an increased consumption of isocyanate groups indicating a higher need of NCO compared with conventional polyurethane systems.

Powder Coatings
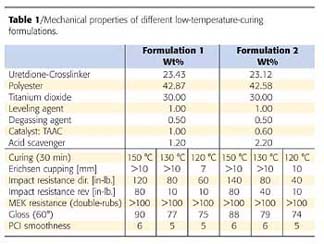
Powder coating formulations are much more complex than a simple model system. They contain a number of ingredients, pigments and additives. The requirements include mechanical performance like flexibility and hardness, and a sufficient crosslink density in terms of chemical resistance. In addition, powder formulations have to show storage stability and at the same time good flow properties, which depend both on the glass transition temperature and are opposing to each other.Table 1 shows powder formulation data.6 The NCO/OH ratio is 1.7:1. Obviously we were able to achieve good mechanical data and chemical resistance at 30 minutes at 130 °C and even lower. The reactivity can be adjusted either by the concentration of the catalyst or of the acid scavenger.
Accelerated weathering tests (QUV-A, QUV-B, weather-o-meter) revealed a weather resistance of this novel low-temperature curing system, which is similar to conventional polyurethane formulation (cured at 200 °C) based on the same composition (Figure 7).6 If superdurable polyester resins are used, however, the weatherability is enhanced significantly. This stresses that the resin, not the hardener nor the catalyst, is the fragile link in this chain.
Summary
Due to special catalysts, it is now possible to decrease the crosslinking temperature of blocking agent-free PU-powder formulations down to 120 °C. Now temperature-sensitive substrates like wood, plastics and pre-assembled parts can be coated by this ecological-compliant technology. To ensure a good flow behavior, tailor-made components with low melt viscosity have to be used. The resulting coating films show excellent mechanical properties and weather resistance.References
1 Pieter Gillis de Lange, "Powder Coating: Chemistry and Technology", Vincentz Verlag, 2004.2 A. Wenning et al. "Polyisocyanates today and tomorrow" Powder Coating Europe 98, Amsterdam, Netherlands, 20-22 January 1998.
3 a) W. Grenda, J-V. Weiß, "Uretdione based Crosslinkers for Powder Coatings", Coating Symposium Powder Coatings Technology, Mulhouse, 2001.
b) W. Grenda, J-V. Weiß, "Polyurethane hardeners for powder coating specialties", Farbe & Lack, 6, (2000), 97.
4 a) E. Spyrou et al. "Isophorone diisocyanate in blocking agent free polyurethane powder coating hardeners", Progress in Organic Coatings, 48, (2003), 201-206.
b) Auf der Heyde et al., Die Angewandte Makromolekulare Chemie, 153, (1987), 1-13.
5 a) E. Spyrou, Farbe & Lack, 106, (2000), 126-130.
b) R. Lomölder, F. Plogmann, P. Speier, JCT, 69, (1997), 868.
c) E. Spyrou, R. Lomölder, XXIV. FATIPEC-Congress, Interlaken, Vol. B, (1998), 159-174.
6 Detailed data have been published recently: W. Grenda, E. Spyrou, J.V. Weiß, "Low temperature cure powder coatings, a new challenge for uretdione based polyurethanes", International Waterborne, High solids and Powder Coatings Symposium, New Orleans, February 2-4, 2005.
7 a) J.M. Loutz et al. "New developments in powder coatings", Powder Coatings, Vol. 183, (1993), No. 4341, 584-593.
b) D. Richart, "Powder Coating: Current Developments, Future Trends", International Waterborne, High solids and Powder Coatings Symposium, New Orleans, Feb. 22-24, 1995.
This paper was presented at the 8th Nürnberg Congress, Creative Advances in Coatings Technology, April 2005 in Nürnberg, Germany. The Congress is sponsored by FPL, PRA and the Vincentz Network.
For more information, e-mail emmanouil.spyrou@degussa.com.


Report Abusive Comment