Highly Flexible and Transparent Nanocomposite Coatings on Compliant Surfaces
This paper reviews the engineering of nanocomposite thin films by adjusting inorganic nanoparticle loadings in a polymer matrix.
Optically transparent solids have numerous applications and have experienced widespread use for centuries. Glass was the predominant material over most of that time, with additives often included to engineer specific properties. A wide range of refractive indices and absorption characteristics were eventually produced. Glass and common metal oxides eventually spanned the refractive index range from 1.44 (SiO2) to 2.7 (TiO2). In the modern era, polymers have become a common alternative to glass in many applications.
Visibly transparent polymers have the advantages of low cost, processing ease and a wide range of physical properties. Additionally, polymers exhibit strain behavior that far exceeds the limits of glass. Urethanes and polycarbonate, in particular, also have very high impact resistance. However, transparent polymers have a more limited range of refractive index than glasses and generally scratch more easily. An inorganic-organic nanocomposite ideally maintains the flexibility, low cost and processability common with organic polymers, while extending other material properties beyond the limits of the host polymer. With proper selection, loading and surface modification, inorganic nanoparticles can be used to significantly broaden the engineering potential of transparent polymers.
Over the past few decades, excitement has built around these hybrid inorganic-organic materials that can offer improved mechanical, optical, thermal and electrical properties. Transparent nanocomposite films on the order of nanometers to microns have successfully been used to improve abrasion resistance, alter reflectance and minimize the UV exposure of substrates. The advantages of these materials include low processing costs, low processing temperatures and extended strain ranges over ceramic films.
The primary optical property of a material is its complex index of refraction, which is an engineering constant that explains the propagation and absorption of electromagnetic waves through materials and at interfaces. The design and production of optical filters is mainly accomplished through the combination of thin films with unique refractive indices.(1) These are typically dielectric and metal films that are almost exclusively deposited using vacuum deposition. Although these materials have been studied extensively, they have significant disadvantages related to the required processing steps and limited mechanical properties of the resultant films.
Nanocomposites composed of inorganic nanoparticles embedded in an organic polymer matrix directly address these issues.(2) The primary challenge for nanocomposites is to alter the optical properties of a material without affecting the visible transparence of the final article. When an electromagnetic wave encounters a boundary between two materials of discrete refractive index, the direction of the wave is altered both through reflection and refraction. This holds true with sub-micron particles, where the optical dimension (refractive index and diameter) must be engineered so as to not scatter light waves (scattering decreases transparence). Thus, a homogenous distribution of discrete, mono-disperse nanoparticles with dimensions less than 1/10 the wavelength of the encountered light must occur within these nanocomposites to ensure high visible transparence.
The focus of our research for the past several years has been spin coating inorganic-organic nanocomposite films that are used on visibly transparent, flexible substrates. The engineering limits of polymers have been extended through incorporation of nanoparticles that make up over half the volume of the composite. Spin coating is practically limited to substrates that cover less than a square meter. It will be required to coat larger areas to move this technology towards broader applications.
Large-area deposition of nanocomposites could more effectively and economically reduce unwanted reflections from consumer products such as cell phones, computers and e-book readers. Inexpensive deposition of thin-film filters over large areas is also applicable to solar energy, where management of the solar spectrum is beneficial (UV and IR reflectors, improved visible transmittance and spectrum splitting). Additionally, these large-area processes can be adapted to three-dimensional deposition, further extending the reach of this technology.
 Credit: Optical Dynamics Credit: Optical Dynamics |
| Figure 1 Click to enlarge |
Nanoparticles
In optical applications, it is crucial to obtain ultrafine and stable nanodispersions in order to produce thin films with low surface roughness and haze. Although the primary size of most commercial nanoparticles is quite small (5-50 nm), the high nanoparticle surface energies cause agglomeration in the synthesis and post-synthesis processes. This leads to dispersions with primary particles in the nanometer range, but with a significant number of large particle groups exhibiting complex shapes and morphologies due to agglomerated and aggregated networks. These conditions negatively impact haze and transparency.(3) The term agglomerates relates to groups with weak interparticle bonds that allow them to be re-dispersed in a solvent. Ball milling and ultrasonication are typically used to break up agglomerates. The term aggregates (or hard agglomerates) describes groups with primary particles held together by strong attractive forces.(4) Aggregates formed during high-temperature synthesis often persist after ultrasonication or milling and must often be removed through centrifugation or filtration.
Solution-based synthesis of nanoparticles was first reported by Stober in 1968(5) using a tetraethoxy silane (TEOS). Many researchers have improved on the original methods, with a key enhancement being modification of particle surfaces with functional organic groups, designated as an ORMOSIL (Organically Modified Silica).(6) This method served as a basis for production of other metal oxide nanoparticles through hydrothermal and solvothermal methods. The solvothermal method is carried out in a closed reactor in which precursors of the metal oxide are mixed into a solvent. The reactants are heated and reaction kinetics are adjusted to alter the size of the nanoparticles. Solution synthesis of nanoparticles has three very important advantages:
- The nanoparticles are grown and harvested in a liquid, reducing airborne contaminants.
- High heats are not required, so the presence of aggregates is reduced.
- The solvents are amenable to several surface functionalization schemes.
Preservation of discrete nanoparticles is of utmost importance for high visible transparence in the finished article. Dispersion of nanoparticles in liquids and solids can be aided by functionalization of the particle surface. Several functionalization schemes have been demonstrated in literature, including the use of ions(7), surfactants, ligands(8), polymers(9), coupling agents(10), and shells of silica(11) or polymer.(12)
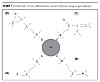
The nanoparticles discussed here are metal oxides, which are subject to simple functionalization using organosilanes (as typically used with ORMOSILS). The nanoparticles can be functionalized with different groups that can include single groups that may or may not be polymerizable (Figure 1). Functionalization that includes a polymerizable group will aid in making the nanoparticles an integral part of the matrix.
Engineered Nanocomposite Films
 Credit: Optical Dynamics Credit: Optical Dynamics |
| Figure 2 Click to enlarge |
The third element of these nanocomposites is the polymer that envelops the particles in a matrix, which may also include covalent bonds between the inorganic and organic phases. Nanoparticles can be dispersed directly into a swelled polymer, although dispersing monomers or oligomers directly into a nanoparticle solution has advantages. Many monomers are soluble in the same solvents the nanoparticles are already stabilized in and can polymerize to functional groups on the particles. Once the solvent has been removed, polymerization of the nanocomposite can be performed using thermal or photoinitiated curing.
The optical and mechanical properties of a nanocomposite are engineered by varying monomers, initiators, curing conditions and the concentration of nanoparticles used in the matrix. With spin coating techniques, up to 65 volume percent nanoparticle loading is possible, which is near the theoretical limit of close packing with spheres. Between the properties of the base polymer and the fully loaded nanocomposite, a continuous realm of possible combinations exists.
Spin coating is a well-understood deposition technique that produces reproducible, uniform films that are spread across a substrate with considerable shear forces. When the optical diameter of the nanoparticles becomes too large, scattering results as light waves are reflected from the boundary of the inorganic and organic phases (Figure 2). A homogeneous dispersion of discrete mono-disperse nanoparticles will yield a highly transparent nanocomposite.
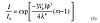 Credit: Optical Dynamics Credit: Optical Dynamics |
| Equation 1 Click to enlarge |
Models predicting light scatter intensity in a nanocomposite show a linear relationship with path length, packing density and refractive index ratio, but scattering varies with the cube of particle diameter.(13) (See Equation 1),where I/I0 is the ratio of transmitted intensity to the initial intensity, Vp is the volume packing density of the nanoparticles, l is the path length, φ is the diameter of the nanoparticle, λ is the wavelength of light, and m is the ratio of the refractive indices at the inorganic to organic boundary.
The refractive index of a nanocomposite is the combination of the volume fraction of the inorganic and organic components. The composite refractive index can be modeled as: (See Equation 2), where ni and vi are the refractive index and volume fractions of the components.14 The inorganic nanoparticles have a non-uniform refractive index across the visible spectrum, whereas the organic polymer is nearly constant.
 Credit: Optical Dynamics Credit: Optical Dynamics |
| Equation 2 Click to enlarge |
 Credit: Optical Dynamics Credit: Optical Dynamics |
| Figure 3 Click to enlarge |
To demonstrate this effect, measurements were made using nanocomposite films approximately 500 nm thick with refractive indices between 1.5 and 1.75 (measured at 480 nm). The range of refractive indices was achieved with 5-50% loading of ZnO nanoparticles in the UV-curable monomer TMPTA. Results are shown in Figure 3. The films were applied to a substrate using an Optical Dynamics spin coater. The source of the ZnO is a nanoparticle dispersion in methyl ethyl ketone (MEK) produced by Umicore (Zano MEK 067). It is reported as containing 30 nm ZnO nanoparticles at 45 weight percent and uses a surfactant to maintain nanoparticle separation. Reflectance was measured with a contact spectrophotometer (F20 by Filmetrics), with thickness and roughness determined using a contact profilometer (XP-1 by Ambios corporation).
This data was then used to determine refractive index using a Cauchy model. It should be noted that the refractive index of the fully loaded ZnO film has been determined to be approximately 1.75, which is slightly lower than the expected 1.82. This is most likely due to the added surfactant reducing the effective refractive index of the ZnO nanoparticles. A similar study of TiO2 nanocomposite films was undertaken and resulted in a maximum refractive index of 1.88 with volume packing of 65 percent. In this case surface modification was responsible for the reduced refractive index. These films were used to produce thin-film reflective filters with up to 38 layers, with the resultant stack surviving strains up to 25 percent.(15)
 Credit: Optical Dynamics Credit: Optical Dynamics |
| Figure 4 Click to enlarge |
There is potential for the use of nanocomposite films in absorptive filters that maintain high visible transparence. A possible use is protecting eyes from harmful ultraviolet or infrared wavelengths. Metal oxides have high absorption in the UV region with low absorption in the visible range, which makes them ideal for optical applications requiring UV blocking. It is well known that titanium dioxide and ZnO have very high absorption in the ultraviolet region. The visible and UV response of 500-nm-thick films containing varying volume ratios of ZnO nanoparticles was measured using a UV-Vis spectrophotometer (8453 by Hewlett Packard). The ZnO dispersions demonstrated sharp changes in response at about 380 nm, as expected when the UV absorbance of ZnO is considered. The UV absorbance (λ = 340 nm) was linearly related to the volume fraction of ZnO in the films (Figure 4), as predicted by equation 1.
A similar study of the impact of volumetric loading of nanoparticles on the modulus of a thin film resulted in a maximum modulus near 60 percent loading.(16) In this study, silica nanoparticles were used at volumes ranging from 30 to 75 percent and the modulus was measured using nanoindentation.
Deposition
The films discussed above were spin coated with equipment that was specifically designed to deposit thin-film nanocomposites. The coater moves up to four 80-mm-diameter substrates through three process steps: cleaning, coating and curing (Figure 5a). The unit maintains a consistent internal temperature of up to 100 oF for evaporation consistency and includes a HEPA filter to reduce film defects. The system is completely programmable and can deposit up to six distinct coating chemistries.
Surfaces to be coated are first cleaned with a high-pressure wash (1000 psi) to remove fine particulates. The substrate is then moved to the coating bowl where one of six chemistries can be applied to the spinning substrate. The liquid coatings are filtered at the nozzle (5-micron filters) and applied to the spinning substrate (~1000 rpm) using computer controlled solenoid valves (Figure 5b). The dispersions are applied from beneath the substrate and readily coat flat, cylindrical, toric or spherical shapes. All of the coating parameters (spin speed, substrate sweep over the dispense nozzle, dispense pressure, dispense time, air flow and air temperature) are computer controlled, and solvent vapors are removed from the coating chamber using a fan. After the coating is applied, the films are cured using a pulsed xenon strobe lamp. The substrate can then go back to the coating bowl for subsequent layers or be returned to the staging area, after which the next substrate can be processed. The system does not require high temperatures or pressures and deposits films ranging from roughly 30-3,000 nm with an accuracy of +/- 5% for each layer.
Spin coating involves the thinning of a liquid chemistry that is spread across a spinning substrate as solvent evaporation leaves behind the solute. This well-understood technique controls film thickness primarily through the viscosity of the solution and the spin speed used during film formation. The repeatability of the process is very high as long as the coating environment is well controlled, since this leads to solvent evaporation rates that are nearly constant. Initial film thickness is set by a balance between the centrifugal forces applied to the film as the substrate spins and viscous forces that increase as evaporation takes place. Once these forces balance, evaporation becomes the primary driver of film thinning. The forces encountered during spin coating are significantly larger than gravitational forces, so coating complex geometries with negligible variation in coating thickness is practical. Additionally, these forces allow extremely high nanoparticle packing densities to be achieved.
Spin coating is not an ideal candidate for large substrates, and thus the engineer is faced with depositing high-volume-density films without a simply applied body force to overcome the thermodynamic surface forces of the nanoparticles. A large-area deposition will still need to overcome the tendency of nanoparticles to agglomerate. The functionalization of the nanoparticle surface should reduce the surface energy and may aid in the self assembly of the nanocomposite. The stabilization techniques used to keep nanoparticles dispersed in a solvent may not translate into a discrete dispersion in the nanocomposite. Systems that rely on ions to maintain nanoparticle separation in an aqueous dispersion will begin to agglomerate as the water is removed. Steric stabilization techniques using surfactants can also create films that are poorly suited for multilayer applications, since these methods can interfere with interlayer adhesion. Ideally, functionalization would reduce the surface energy of the nanoparticles to a level comparable to that of the monomers used in the system, thus creating a bulk nanocomposite monomer.
A demonstration of a bulk nanocomposite using organosilane functionalization techniques reducing the surface energy of the nanoparticles to achieve a homogeneous dispersion is shown in Figure 6. Two mixtures were created that use alcohol-dispersed silica nanoparticles (Nissan Chemistries IPA-ST) at 10 volume percent in TMPTA. In the first mixture the silica dispersion was used as supplied. The second mixture was functionalized using a methacryloxypropyl trimethoxysilane. The mixtures were then placed into a rotary evaporator and the alcohol was removed and the bulk monomer nanocomposite was cured using UV radiation. The nanoparticles that were not functionalized tended to agglomerate in the polymer matrix, which created haze. The nanoparticles that were functionalized remained separated in the dispersion as shown.
An ideal application method for coatings involving large areas is dip coating. A simple setup was built to pull a glass slide out of a nanoparticle-based coating solution at speeds between 1 and 25 mm/s. The nanocomposite dispersion was cerium dioxide, which is available as a colloidal suspension from Sigma-Aldrich (Product No 289744), and a trimethylolpropane triacrylate. The ceria dispersion was functionalized such that acrylate groups surrounded the nanoparticles. The total volume of nanoparticles in the resultant film was 40 percent. The thickness of the coating was determined to be 270 nm, with a refractive index of 1.8 (measured at 480 nm). The original formulation was then diluted to produce a film on the order of a quarter wavelength (approximately 70 nm), which is shown in Figure 7b. This quarter wave producing high-index chemistry was used along with an SiO2-bearing nanocomposite to produce a 9-layer reflective stack based on an alternating low/high pattern (with curing between dip coating steps). The resultant film is shown in Figure 7a.
Another technique suitable for roll-to-roll coating is spray deposition using the setup shown schematically in Figure 8. A trial was run depositing a nanocomposite with approximately 40 percent nanoparticles by volume. A spray nozzle and micro dispense valve from Lee Electro-Fluidic Systems were used to spray the chemistry. The valve was driven at a frequency of 20 Hz, and a substrate was slowly passed under the nozzle to produce a continuous film across. The resulting coating was highly transparent at a thickness of 2.5 microns. The refractive index of the coating was determined to be approximately 1.75.
Conclusion
In this paper we reviewed the engineering of nanocomposite thin films by adjusting inorganic nanoparticle loadings in a polymer matrix. The nanocomposite films were engineered for refractive index, absorbance and modulus. The preliminary work focused on spin coating techniques in which optical and mechanical properties were engineered with nanoparticles composing nearly 65 percent of the volume. In order to move the technology to large-area deposition and increase applicability to additional industries, nanoparticle surface modification is essential to equalize the surface energies of the nanoparticles and surrounding monomers. Thus, large body forces are not required to overcome the tendency of the nanoparticles to agglomerate. Three systems were shown demonstrating that highly packed nanocomposites can be formed using scalable deposition techniques.
Acknowledgements
This work was supported in part by the National Science Foundation SBIR Phase II award No. 0848825 and in part by an award by the Kentucky Cabinet for Economic Development, Department of Commercialization and Innovation, under the grant agreement KSTC-184-512-09-069 with the Kentucky Science and Technology Corporation.
For more information, visit www.opticaldynamics.com. This paper was presented at the American Coatings Conference, Charlotte, NC, April, 2010.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!
