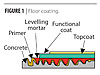
|
| Figure 1 Click to enlarge |
Waterborne polyurethanes with ultra-low VOCs, specifically designed for concrete floor coating applications, have recently been developed with performance properties approaching solventborne polyurethanes.3 This development has led us to wonder if waterborne polyurethane coatings could be used to function as graffiti-resistant coatings for concrete substrates. Additionally, these 2-component waterborne polyurethane topcoats would allow for graffiti resistance in a matte finish – something that the construction market is looking for.
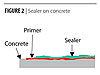
|
| Figure 2 Click to enlarge |
This paper describes the development of waterborne polyurethane coatings that combine good cleanability in an environmentally acceptable system.
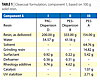
|
| Table 1 Click to enlarge |
Study of Graffiti Resistance
Clearcoat formulations that were tested consisted of resins plus solvent/water and additives. For comparison purposes, commercial resins (II & III in Table 1) were used: solventborne Polyester A/HDI-Biuret 1 and Polyester-Waterborne Dispersion B/HDI-Trimer 1. The Polyacrylic (PAC)-Waterborne Dispersion D was crosslinked with a new component A (HDI Trimer 2) (Table 1, Formulation I).

|
| Table 2 Click to enlarge |
The film properties of I and II were not up to the standards of the solventborne system III, so another series of experiments was run to check these results and expand the study. Table 2 lists the tested formulations and their characteristics. For Formulations I, II and IV we used the “normal” crosslinking ratio for PU topcoats, 150% for waterborne and 110% for solventborne. Formulation II is a variation of Formulation I. The crosslinking ratio used here is 300% instead of 150%. The theory behind this modification was to increase hardness and chemical resistance without giving up application properties. The application viscosity for Formulations I and II was adjusted by adding additional water to the clearcoat. Test panels were coated by roller at a dry film thickness of 3 mils and allowed to cure 7 days before testing.

|
| Table 3 Click to enlarge |
Formulations I through IV from Table 2 give films that are easily cleanable even though two of these formulations are glossy and two are non-glossy. Easily cleanable is described in ASTM method D 6578.4 In this method a series of materials, typically used as graffiti markings, is applied to test panels of the surface being evaluated. The graffiti markings are removed using a series of procedures that begin with wiping with a dry cloth and end with cleaning the surface with an aggressive cleaner. The graffiti resistance is reported as a cleanability level based on the first method, which completely removes the graffiti marking in 25 rubs or less.

|
| Figure 3 Click to enlarge |
We decided to explore more products using the stereochemistry of Formulation II from Table 2. Formulations were prepared with four waterborne systems and a standard solventborne system to explore differences in film properties. These five systems are described in Table 3.

|
| Figure 4 Click to enlarge |
The gloss of these coatings can be controlled by varying the ratio of two hydroxyl-functional resins as shown in Figure 3. For instance Formulation 3 represents the left most points on the graph while Formulation 2 is represented by samples on the right side with 60° gloss less than 10.
Formulations 1 and 3 make glossy films and have the expected good graffiti resistance. Formulation 2 has a matte finish but has surprisingly good graffiti resistance as seen in Figure 4. The epoxy graffiti paint was removed by a water-based citrus cleaner so stronger solvents were not needed.

|
| Figure 5 Click to enlarge |
Formulations 4 and 5 have a matte finish but have poor graffiti resistance. The cleanability of Formulation 5 is shown in Figure 5; the epoxy graffiti paint could not be removed even with MEK, which is the strongest solvent used for this test. The lack of gloss in Formulation 5 was unexpected because it makes a glossy finish when formulated at a higher VOC level. We believe this is caused by differences in coalescence and will be discussed later in this paper.
Surface Energy Study
Surface Roughness Study
The five formulations were examined by atomic force microscopy (AFM) to study the surface morphology of each material. As you can see from the scans, Formulations 1 and 3 show smooth surfaces, which is to be expected from glossy films. Formulation 1 is delivered from solvent, which allows the two components to intermingle before being applied to the surface. This improves coalescence and results in a glossy film as seen in Figure 6.

|
| Figure 6 Click to enlarge |
Formulation 3 gives a glossy and easily cleaned surface even though it is delivered from water. This is because it is a highly crosslinked two-component urethane. It is able to coalesce well because the components are low in viscosity and sufficiently low in reactivity to give them the mobility that allows the polymer hard segments to align on the surface providing a glossy, cleanable surface. This surface is seen in Figure 7.
Formulation 2 (from Table 3) gives a matte finish coating that is easily cleaned. The AFM scan shows the surface is rough (Figure 8), which is expected in a matte finish film. The rough surface diffracts light in different directions so that it does not appear glossy. This AFM scan shows that the surface roughness is worse than Formulations 1 and 3.

|
| Figure 7 Click to enlarge |
Of the five samples, Formulation 4 has the highest surface roughness and Formulation 5 has the second highest in addition to having more obvious surface imperfections. We theorize that this surface roughness allows subsequent paint layers (graffiti) to adhere well to the surface, which makes it harder to be removed with cleaning. Formulation 2 has less surface roughness and contains no obvious pores that give anchor points to further paint layers. This makes Formulation 2 easier to clean.
Results and Discussion
The most important value that can be taken from the AFM study is the roughness value. Table 5 shows the roughness value of each formulation.

|
| Figure 8 Click to enlarge |
We believe that the difference in surface roughness is caused by the ability of each formulation to coalesce in an appropriate amount of time.
Film formation in aqueous systems can be demonstrated by Figure 11. This shows that the material has to go from being discreet particles to a continuous film as the water evaporates. In a two-component system some of the initial particles contain both components, some particles are solely OH, and others are solely polyisocyanate. This variety of particles makes the coalescence more complicated. The coalescence process is competing with the reaction of polyisocyanate with hydroxyl functionality.

|
| Figure 9 Click to enlarge |
Formulation 3 coalesces very slowly, which allows it time to make a glossy film. Formulation 2 coalesces slowly also but is not glossy because it has a combination of two polyacrylate hydroxyl components that are somewhat incompatible and, therefore, the hard segments do not align as they do in a glossy film. It does however form a film with no obvious pores and not enough surface roughness to allow subsequent paint layers to stick to it. We believe that this formulation reaches the delicate balance of being rough enough to diffract light while not being rough enough to provide pores that would serve as anchor points to subsequent paint layers.

|
| Figure 10 Click to enlarge |
Conclusions
It is possible to make coatings that adhere well to concrete, provide a non-glossy surface that is also resistant to graffiti. Easy clean-up has been demonstrated on these systems. It has been shown through AFM that the surface roughness must be somewhere between 10 and 300 nm to give this surface. A balance of incompatible hard segments combined with the proper reaction rate and coalescence speed is required to get matte coatings with outstanding graffiti resistance.
This paper was presented at The Waterborne Symposium, sponsored by The University of Southern Mississippi School of Polymers and High Performance Materials and the Southern Society for Coatings Technology, 2009, New Orleans, LA.

|
| Figure 11 Click to enlarge |





Report Abusive Comment