Zero-VOC and High-Solid-Content Photocured Siliconized Coatings
Technology to Impart Superior Surface Characteristics in Light-Cured Coatings

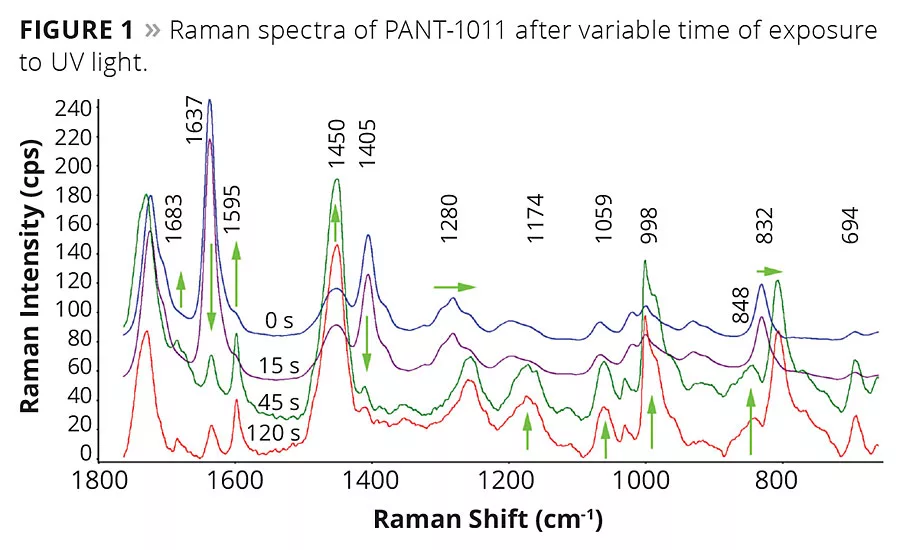
figure 1
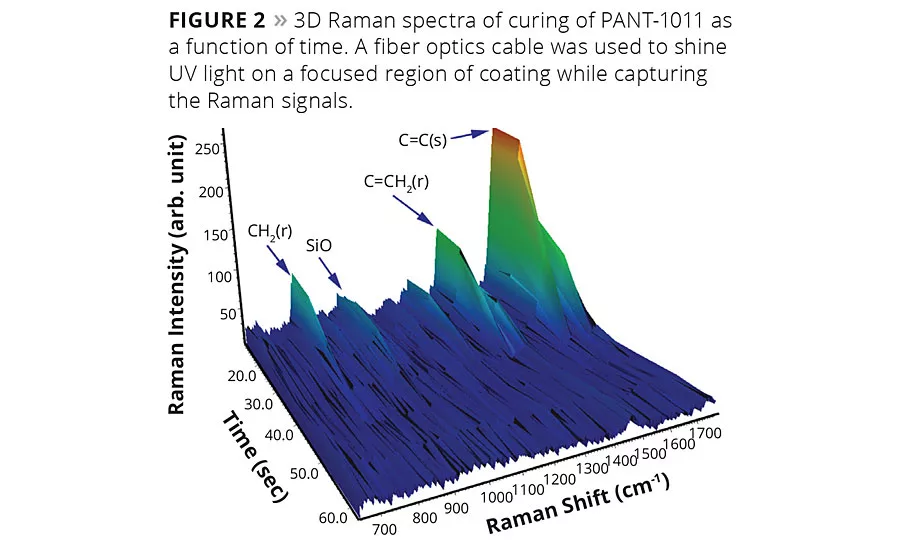
figure 2
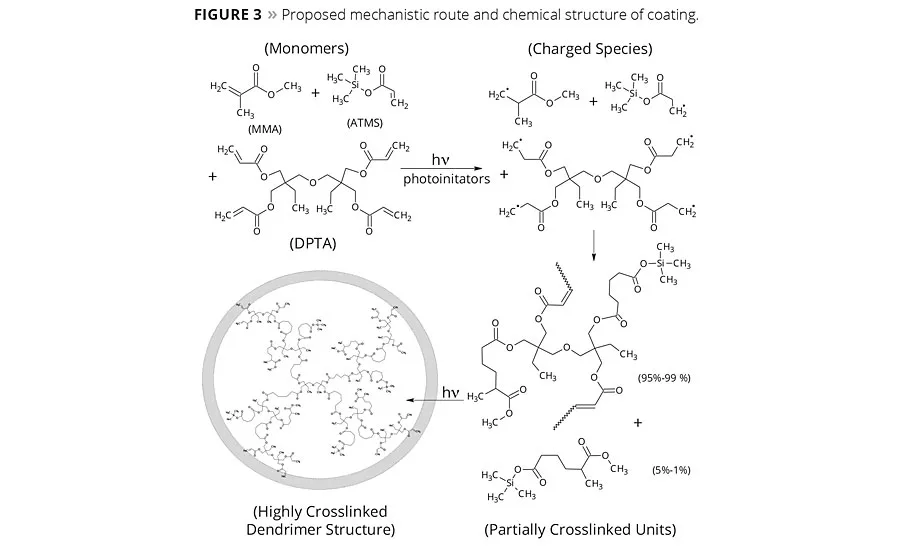
figure 3

figure 4
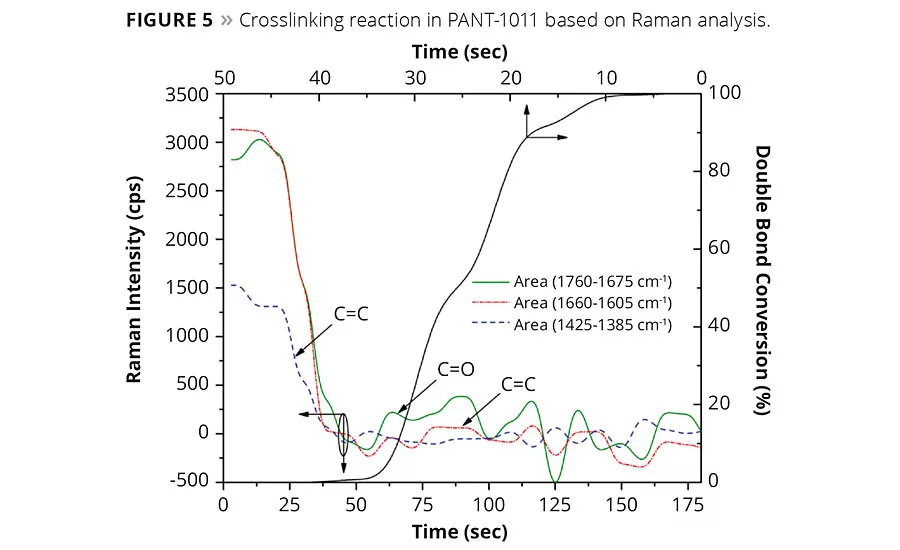
figure 5
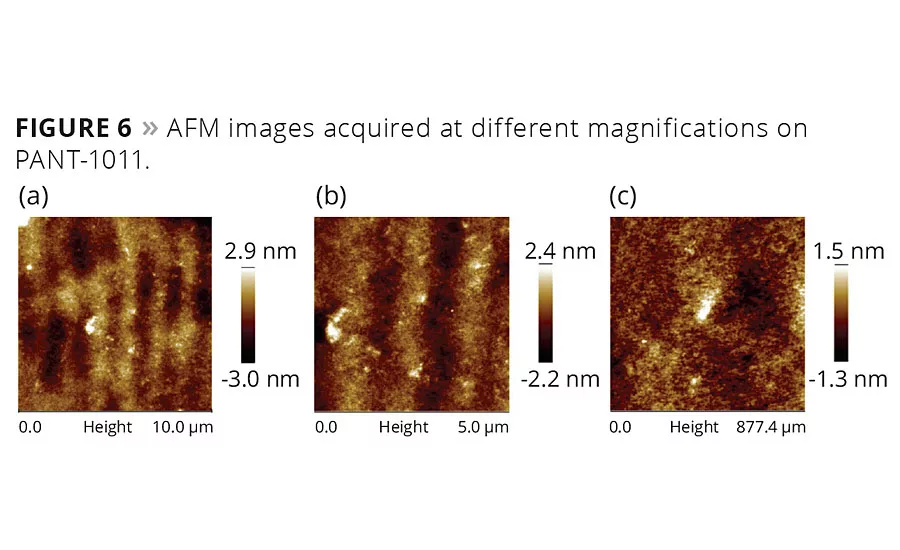
figure 6
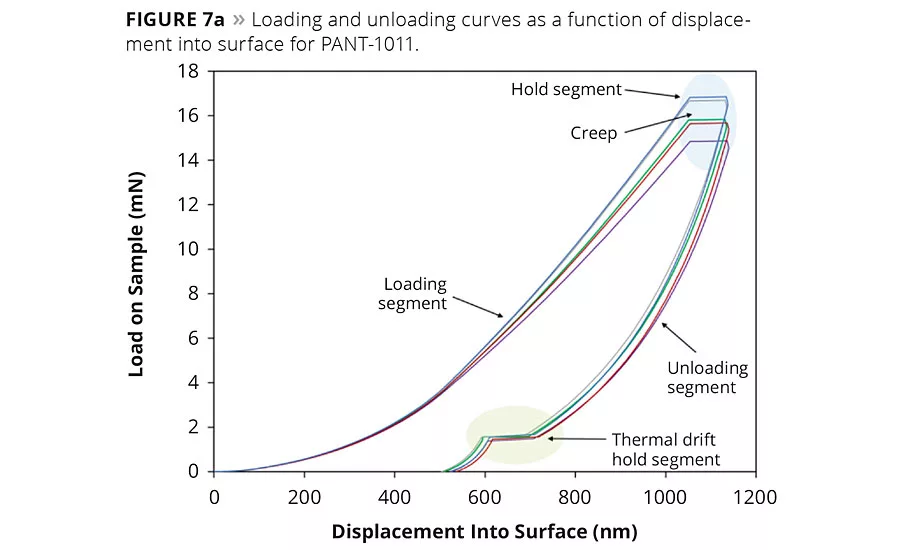
figure 7a

figure 7b

figure 8a
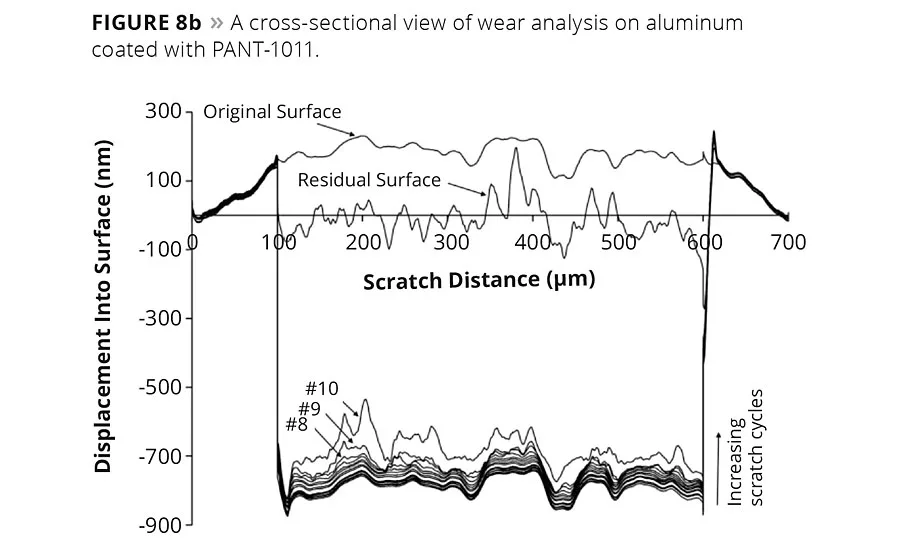
figure 8b

figure 9a

figure 9b
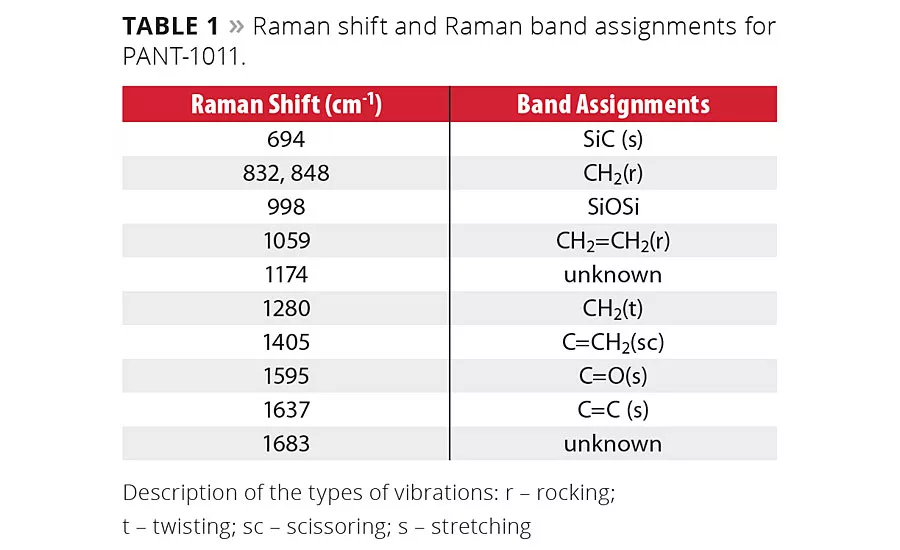
table 1
The Registration Evaluation Authorization and Restriction of Chemicals (REACH) regulation in Europe and regulations imposed by the Environmental Protection Agency (EPA) in the United States have motivated scientists and technicians to adopt safer routes for developing coating formulations. In the coatings industry, UV light-cured coatings (UVCs) have enjoyed tremendous success in the past few decades. UVCs are gaining incredible attention due to inherited benefits such as energy efficiency, low work space requirements, fast and ambient curing temperatures, minimized VOC emissions and superior surface attributes. Although introduced in the early 70s, UVC technology is still evolving, with new commercial products appearing among conventional polymer-based coating products. Numerous curing techniques involving different photoinitiators (PIs) and crosslinkers have been postulated to negate the general perception in the coatings industry that low-temperature curing/hardening means inferior crosslinking and coating strength. The free radical class of PIs has been widely accepted among commercially available PIs, as these can be used with both acrylates and unsaturated polyesters. Similarly, multi-functional low-, medium- and high-molecular-weight acrylates have been introduced to impart the desired level of crosslinking in UVCs. The addition of telechelic silicones in UVC compositions deemed to provide adequate crosslinking anchors along with a flexible inorganic segment. This article explores the possibility of using telechelic silicones in traditional acrylate-based UVC compositions.
An in-depth analysis of cure kinetics of UVCs was conducted using Raman spectroscopic techniques. The surface morphology of the UVC coated substrate was studied with atomic force microscopy (AFM). The hardness and modulus of the siliconized UVC was determined by nanoindentation techniques. Moreover, instrumented nanoscratch tests helped in understanding the adhesive and cohesive modes of failures in siliconized UVC. Attempts have also been made to understand the wear characteristics of the siliconized surface. It is expected that this article will provide readers a good understanding on the properties of siliconized UVC formulations.
Introduction
UV light-induced curing involves a light source that emits photons of a particular wavelength range, unsaturated polymer and PIs. When mixed in a certain ratio, PIs absorb the UV energy from the light source and trigger the chemical reaction that propels the crosslinking reaction between unsaturated bonds. The curing or crosslinking can be accomplished within a fraction of seconds. The time to cure, however, depends on the PIs and other interfering ingredients such as pigments. A wide variety of low-molecular-weight monomers or medium- to high-molecular-weight unsaturated polymers or oligomers are available for the formulation. Acrylates and unsaturated polyesters are the most suitable candidates for such reactions. Unsaturated monomers or oligomers mostly act as reactive diluents in the formulation to manipulate the viscosity or flow characteristic of the formula. In addition to unsaturated monomers and polymers, PIs play a vital role in UV light-assisted curing. Wide varieties of cationic or free radical-generating PIs are available in solid and liquid form to suit formulation requirements. Coating thickness, presence of fillers and type of energy source significantly influence the selection of the PIs for a curing process. The concentration of PIs needs to be optimized for superior curing, as a low amount of PIs will result in inadequate hardening while a higher amount would lead to unwanted shielding of monomers.
Present Scenario
Dedicated efforts have been rendered towards the development of novel coating compositions that can be cured with UV light. Numerous new UV light-curable materials are now commercially available for a wide variety of applications like graphic arts, adhesives and sealants, barrier coatings, release coatings, UV powder coatings, corrosion protection coatings, automotive refinishes, can coatings, photoresists, stereolithography, optics and electronics, photovoltaics, nail polish, dental work and other biomaterials.1 In literature, the development of acrylic melamine resin containing silicone was mentioned by Nishino et al.2 These authors developed the polymer by first reacting acryloloxy ethyl isocyanate with carbinol-modified polydimethylsiloxane and finally with melamine and formaldehyde in the presence of methyl ether of hydro quinone inhibitor and para-toulenesulfonic acid catalyst. A highly transparent polymer was achieved that displayed an elevated hardness value and an approximately 97°contact angle value. Shang et al.3 prepared an acrylate dispersion containing silica sol that was obtained from tetra-ethoxysilane. On being subjected to a flame test the coated wood substrate extinguished immediately, suggesting that the presence of silicone can improve flame retardant characteristics. In another interesting study a tri-functional acrylate monomer was combined with silanes having different chemical structures to form UV-curable coating compositions.4 It was discovered that the addition of silicones modified the surface properties of the coating on curing. The contact angle in this case varied significantly and was dependent on the type of silicone in the formulation. The combination of fluorinated acrylated monomer along with silicone could lead to significantly important coatings. Park et al.5 reported a UV-curable fluorinated polyurethane acrylate coating composition containing vinyl-terminated silicone. It was demonstrated that with an increase in the amount of silicone the storage modulus in this coating increased significantly, while elongation at break decreased. Bowman et al.6 invented a low-gloss coating composition containing polyene, polythiol and a flattening agent along with coloring pigment that can be cured with multiple UV radiation dosages. Inventors used a UV LED lamp (with wavelengths of 365 nm and 395 nm) that was placed between 4 to 20 inches from the substrates. Similarly, Bishop et al.7 demonstrated a patent-pending technology for the development of LED light-cured coating compositions made of urethane acrylate oligomer, a reactive diluent and photoinitiator. Inventors utilized LED light having a wavelength from 100 nm to 900 nm to cure this coating on optical fibers. Chatterjee et al.8 developed a patent-pending pressure-sensitive adhesive and release coating comprised of an organosilicone compound, a halomethyl-1,3,5-triazine and silicate tackifier. Tanaka et al.9 described a patent-pending radiation-curable silicone composition. Their composition contained epoxy-functionalized cation polymerizable organopolysiloxane along with iodonium salt having a cation moiety acting as photoinitiator. Fu et al.10 developed a patent-pending UV-curable silicone composition that can be used as an antidust coating. The coating composition contained an organosilicone having multiple oxirane rings and a cationic photocatalyst solution such as bis(4-alkylaryl) iodonium hexafluoroantimonate salt plus photosensitizer in a glycidyl ether reactive diluent. Vanmeulder et al.11 developed a patent-pending aqueous radiation-curable coating composition comprised of a radiation-curable polymer and an organic matting polymer. A commercial radiation-curable polyurethane dispersion was combined with photoinitiator 1-hydroxy-cyclohexyl-phenyl-ketone, a polyethylene wax dispersion, wetting agent, antifoaming agent and anionic acrylic polymer to make an aqueous dispersion.
Methodologies
Precursor Preparation
The prepolymers and monomers were freed from inhibitors by passing them through a column of inhibitor removal beads. To produce PANT-1011 a calculated quantity of di(trimethylolpropane) tetraacrylate was diluted with methyl methacrylate and variable amounts of acryloxytrimethylsilane. The reaction mixture was homogenized for 30 min and charged with IRGACURE 1173 or IRGACURE 184. To produce PANT-1012, the above composition was charged with Laromer 9026, BYK 3505, IRGACURE 819 and IRGACURE 2022 and Irgazin®Orange EH 1287 pigment.
Spray Application
Q-Lab 3x6-inch steel panels were prepared with PreKote®surface pretreatment from Pantheon Chemical Inc. and left to dry in ambient conditions before coating application. The panels were spray coated using a Porter Cable PSH2 gravity feed detail spray gun operating at 50 psi air pressure. To cure, the coated panels were exposed to a 400-watt Loctite metal halide UV light lamp for 60 sec.
Raman Spectroscopy Characterization
Raman spectra were recorded on a Thermo Electron Nicolet Almega XR dispersive Raman spectrometer. Spectra of the desired region were acquired using an Atlus microscope and Omnic software. Metal coupons were coated and analyzed directly under a dispersive Raman spectrophotometer using an integrated Atlus optical microscope.
Morphological Characteristics
The atomic force microscopic (AFM) technique was used to study the surface morphology of the coatings hardened over a polished aluminum surface. The Bruker Multimode IV operating in ScanAssist mode was used to capture the surface topography.
Nanomechanical Properties
The Al6061-T6 specimens were cut into 1x1 cm2 squares and adhered to circular aluminum stubs using thermoplastic polymer. The specimens were then polished using a 0.05 µm aluminum oxide agglomerate solution and dried until coated. The homogeneous solution of PANT-1011 was applied on the above polished aluminum specimens and cured in ambient conditions by exposing them to a UV light lamp for 60 sec. The coated specimens were left in ambient conditions for seven days before testing their nanomechanical properties.
The nanomechanical analysis was conducted on a MTS Nanoindenter XP with a Berkovich diamond tip. The hardness (H) and modulus (E) were measured using the continuous stiffness measurement (CSM) option. Fused silica was used as a standard calibration sample. The coated specimens were then mounted on the Nan-oindenter XP nanopositioning tray and tested using dynamic contact module (DCM). The “DCM-CSM standard hardness, modulus and tip calibration” test method was utilized for the analysis. The H and E were calculated from 1/10 of the film thickness. A Berkovich tip was used to perform between six to twelve tests on each sample in different regions to achieve a reasonable representation of the nanomechanical properties of the coatings. The nano-indentation data was acquired with the help of TestWork 4 software from MTS instruments. The TestWork software exported the raw data files to the MS Excel software. The Excel data sheets were then imported on Analyst software from the MTS instrument for the data reduction. The Analyst-Excel files were finally used to plot the curves using Origin 7.5 software.
Results and Discussion
Curing Characteristics
The chemical structural features inherent in the UVC compositions can be monitored effectively using Raman spectroscopy. The spectral assignments shown in Figure 1 were acquired on PANT-1011 with variable UV light exposure time. Significant changes in spectral peak assignments were recorded as a function of time of exposure. As the reaction was carried out in ambient conditions and in the presence of oxygen, not much difference was observed in the first 15 sec of exposure to UV light. The changes were apparent after 45 sec of UV light exposure. The peak assignments are displayed in Table 1, and changes in peaks are shown with arrows in Figure 1. The peak intensity at 1637 cm-1 representing unsaturated double bonds stretching vibrations decreased significantly, making the carbonyl peak visibly strong. Similarly, another peak at 1405 cm-1 representing scissoring vibrations of unsaturation decreased with time. These unsaturated bonds open in the presence of photoinitiators to form a crosslinking network. The peak position at 1280 cm-1 and 832 cm-1 shifted significantly due to changed chemical environment after the crosslinking reaction. The 3D plot of Raman signals acquired as a function of time using fiber optics UV light point source is shown in Figure 2. The consumption of C=C during the crosslinking reaction is clearly evident in the spectra.
Reactants and Products
The reactive ingredients were chosen to follow the free radical polymerization mechanism. The initial step in curing through a photocurable free radical system involves the formation of free radical species on absorption of light by PIs. The active radical entity attacks on unsaturated monomers or oligomers (initiation) and induces the chain growth polymerization (propagation) or crosslinking reaction. The reaction proceeds until most of the unsaturation is consumed (termination), leading to a near-saturated, dense network. The chemical route and possible coating structure is shown in Figure 3, and the key PIs for free radical polymerization are displayed in Figure 4. The extent of crosslinking in compound as a function of time can be derived from the Raman band assignments. Figure 5 displays the consumption of double bonds in the formation of a crosslinked network as derived from Raman spectral assignments. Apparently, the carbonyl group in the vicinity of unsaturation also diminishes as the reaction proceeds. The percentage conversion curve suggested that almost complete curing was accomplished in approximately 60 sec of exposure. The physical and chemical properties of the coating will ultimately depend on the extent of the main reaction and side reactions shown in Figure 3.
Morphological Features
The coating surface was transparent and hard to analyze under a conventional microscope. AFM was therefore used to look into the inherent features of the coating. An alternate bright and dark rib-like pattern was observed on the surface (Figure 6a), probably due to the presence of a thin coating in the grooves of the metal surface as a result of the polishing step. The brighter and dark regimes were fairly miscible and coexisted. There were few scattered bright spots, probably from the clustering of unreacted monomers or leftover PIs. At higher magnification (Figure 6b), it was apparent that darker spots were from unreacted acrylates, while the bright particles were PIs that remained unutilized in reaction. On further magnification down to 877 nm, the nanoscopic level phase separation was evident (Figure 6c). Based on these observations, it can be stated that PIs either did not dissolve uniformly and distributed homogenously at the submicron level, or the amount of PIs exceeded the reaction requirement.
Nanomechanical Analysis
The plots of load on the sample as a function of displacement into the surface of PANT-1011 coatings are shown in Figure 7a. The loading and unloading curves obtained over coating surfaces suggested that both the elastic and plastic domains coexisted in the material. Moreover, the loading and unloading curves did not show any inconsistency, which negates the possibility of fracture-induced failure in the coating. As mentioned earlier, CSM mode was used to collect data to enable extracting H and E values continuously over time. A noticeable amount of elastic behavior was prominent from the curves, probably due to the presence of silicone in the backbone network. The coating displayed creep phenomenon during the hold segment, which is typical for hydrocarbon-based materials. The H and E values are plotted as a function of displacement into the surface and shown in Figure 7b. The H and E values were derived from 10% of the coating’s thickness, and substrate effect is evident in the E curve beyond 300 nm surface penetration. The average H value was 0.78 GPa, which is significantly higher than those observed in our previous studies on hybrid coatings.12 A significantly high E value (12.5 GPa) was observed in the material, suggesting a higher compliance in the coated system.
Cohesion and adhesion between the coating and metal surface can be gauged with nanoscratch tests using nanoindentation techniques. Figure 8a shows a stitched optical image of a nanoscratch test performed on PANT-1011, and also demonstrates the penetration of a nanoindenter head during a nanoscratch test as a function of scratch distance. The original surface morphology, residual surface morphology after the scratch, and critical load value are shown on the curve. Originally, the surface had an average roughness of approximately 225 nm, which increased to a significant value after the indenter tip penetrated to 1700 nm. The critical load to failure is the point where residual morphology apparently differs from the original morphology. The indenter head slid on the coating until 390 µm before creating a fracture at the critical load of 14 mN. The PANT-1011-coated coupons were also subjected to 8 mN wear load for 15 cycles using a Berkovich tip in nanoindenter (Figure 8b). The coating surface was scanned prior to and after the end of the wear cycles. PANT-1011 did not show signs of deformation until 13 cycles, as little damage was observed in 14th wear cycle. The maximum wear track deformation that occurred in the final wear cycle (#15) was approximately 430 m2, where the average displacement into the surface was approximately 670 nm. This recorded wear deformation in PANT-1011 is significantly less than that observed in high-solid-content quasiceramic coatings.13-14 The average friction coefficient during the entire wear analysis was 6.7, while the average lateral force was 5.6 mN.
Application Demonstration
Two sets of panels were selected for the surface planarization and coating adhesion demonstration. A set of panels was coated with Rust-Oleum clean metal primer prior to coating with PANT-1011 and PANT-1012. Figure 9a shows two variations of coatings i.e., pristine PANT-1011 and pigmented PANT-1012. Coatings were spray coated and dried by exposing the surface to UV light for 60 sec. Figure 9b shows a representation of the PANT-coated panels. Crosshead scratches and pull adhesion tests are being conducted, along with a corrosion test per ASTM B117, and results will be published in the next part of this article.
Conclusion
This article describes the development of siliconized UV light-cured coatings. The Raman spectroscopic analysis helped in understanding the crosslinking and hardening reaction in the material. The AFM analysis showed the surface morphological features and distribution of PIs in the coating structure. Several nanomechanical analyses were performed on a thin coating hardened over an aluminum coupon. The H and E values were noticeably higher compared to similar coatings. The nanoscratch and wear analysis on the coating displayed the material’s high cohesive and adhesive strength. It can be summarized that the addition of an adequate amount of silicone in the backbone structure can lead to significant improvements in the surface properties of coatings. n
References
1 Polykarpov, A.; Tiwari, A. Photocured Materials: A General Perspective., Photocured Materials, Royal Society of Chemistry: London, 2015; p 1-14.
2 Nishino, G.; Kanda, S.; Sugimoto, H.; Inomata, K.; Nakanishi, E. Preparation and Coating Properties of an Acrylic Melamine Resin Containing Silicone Segments. Polymer Bulletin2012, 68 (7), 2049-2060.
3 Shang, D.; Sun, X.; Hang, J.; Jin, L.; Shi, L. Preparation and Stability of Silica Sol/TPGDA Dispersions and its Application in the UV-Curable Hybrid Coatings for Fire Protection. Journal of Sol-Gel Science and Technology2013, 67 (1), 39-49.
4 Mohseni, M.; Bastani, S.; Jannesari, A. Influence of Silane Structure on Curing Behavior and Surface Properties of Sol–Gel Based UV-Curable Organic–Inorganic Hybrid Coatings. Progress in Organic Coatings2014, 77 (7), 1191-1199.
5 Park, J.; Jeon, J.; Lee, Y.; Lee, D.; Park, H.; Chun, H.; Do Kim, H. Synthesis and Properties of UV-Curable Polyurethane Acrylates Containing Fluorinated Acrylic Monomer/Vinyltrimethoxysilane. Polymer Bulletin2015, 72 (8), 1921-1936.
6 Bowman, M.; Muschar, H. L. Low-Gloss UV-Cured Coatings for Aircraft. US8906468B2, 2015.
7 Bishop, T.; Gan, K. Led Curing of Radiation-Curable Optical Fiber Coating Compositions. US20150072144 A1, 2015.
8 Chatterjee, J.; Lee, H. S.; Purgett, M. D.; Rathore, J. S. UV-Curable Silicone Release Compositions. US20120196122 A1, 2015.
9 Tanaka, K.; Irifune, S. Radiation-Curable Silicone Composition. US20100255417 A1, 2010.
10Fu, P. F.; Moyer, E.; Rong, W. UV-Curable Silicone Compositions and Anti-Dust Coating Compositions Containing same. WO2015100258 A1, 2015.
11Vanmeulder, G.; Salviato, J. Y. Aqueous Radiation-Curable Coating Compostions. US9062212 B2, 2015.
12 Tiwari, A.; Hihara, L. H. High-Performance Reaction-Induced Quasi-Ceramic Silicone Conversion Coating for Corrosion Protection of Aluminium Alloys. Progress in Organic Coatings2010, 69 (1), 16-25.
13 Tiwari, A.; Liskiewicz, T. W.; Hihara, L. H. Studies of Wear and Tear and Hydrogen Bonding in Dendrimeric Fluoro-Polysilsequioxanes Coatings on Aluminium Surface, Industrial & Engineering Chemistry Research2016, (In Press).
14Tiwari, A.; Hihara, L. H. Chapter 10 - Sol-Gel Route for the Development of Smart Green Conversion Coatings for Corrosion Protection of Metal Alloys. In Intelligent Coatings for Corrosion Control, Butterworth-Heinemann: Boston, 2015, pp 363-407.
Acknowledgement
The author would like to thank the director of the Hawaii Corrosion Laboratory, University of Hawaii at Manoa, USA, for the encouragement and help in writing this manuscript. I would also like to thank the CEO of Pantheon Chemical Inc., Ms. Laura Roberts, for her support during the compilation of this study.
For more information, email atiwari@pantheonchemical.com.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!





