Environmentally Friendly Coatings: Historical Perspectives and Future Outlook

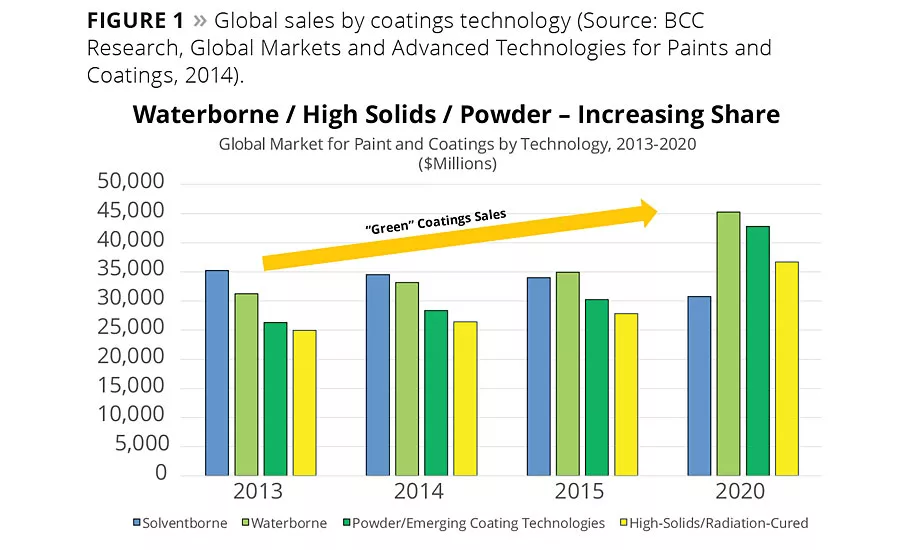
Figure 1
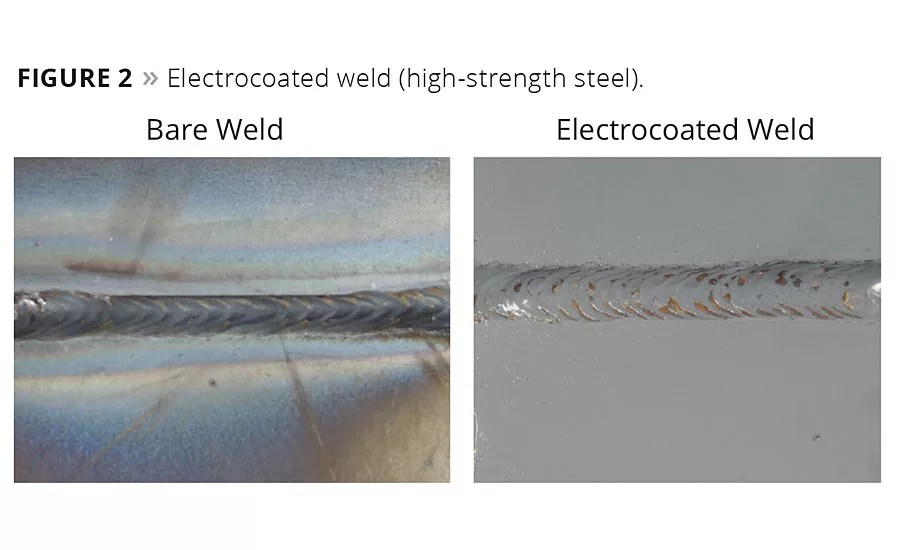
Figure 2

Figure 3

Figure 4
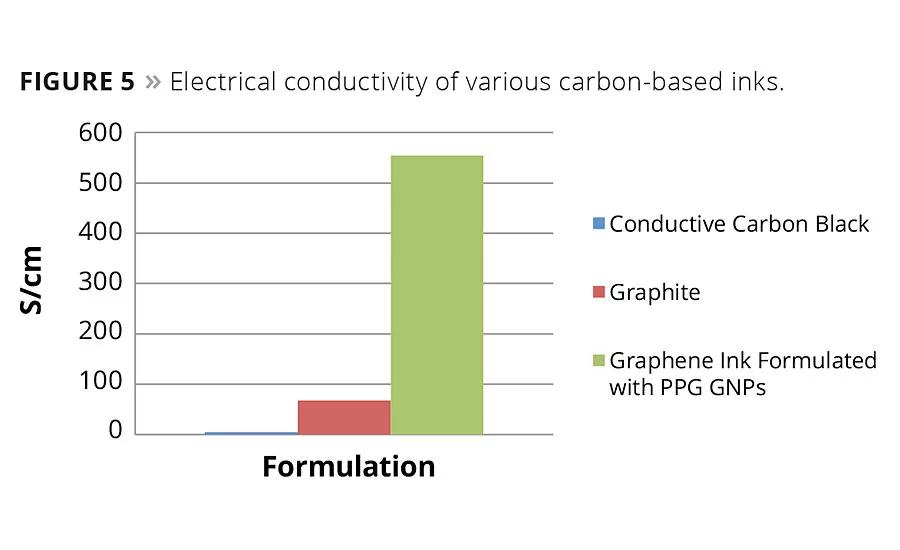
Figure 5
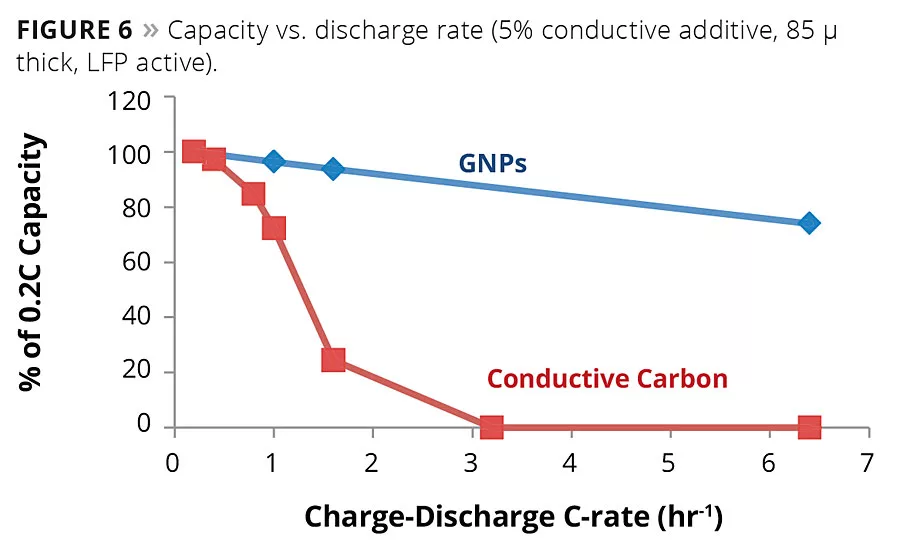
Figure 6

Figure 7
Coatings are amazing materials. While only several thousandths of an inch thick, they are expected to protect and beautify various substrates for many years in various aggressive environments. Unfortunately, the coatings industry has a reputation for causing environmental damage during the manufacture, application and use of products. The combined efforts of global regulatory agencies and coatings technologists have dramatically improved the environmental footprint of the coatings industry over the last several decades. Significant reductions in hazardous air pollutants, releases of volatile organic compounds (VOCs) and hazardous waste have been realized. In the United States, the coatings sector’s toxic releases have decreased more than 80% over the past 20 years. Production waste has been reduced by 48%, and 97% of waste solvents are reclaimed for future use. More than 90% of architectural paints sold in the United States are now waterborne. The total quantity of electricity used to produce coatings decreased by 18% in the last 5 years.1 Unused paint recycling is becoming more commonplace through the recycling efforts of Paint Care, Inc. and the American Coatings Association.2 The global emission of organic solvents from applied coatings has been significantly reduced. Automobile corrosion is much improved relative to that which occurred 40 years ago as a result of increased use of galvanized metal and electrodeposition. Ten- to 20-year vehicle longevity is now commonplace. Ships now can glide effortlessly through the water without the drag caused by barnacles and other marine organisms without the use of tin compounds as coating components. Cool roof coatings reflect infrared energy, enabling energy conservation in buildings.3
Indeed, coatings technologists have enabled great strides in the reduction of pollution and the conservation of energy in the recent past. However, much work remains to be done. Some of the planet’s most challenging issues will be impacted by coatings technology in the future. Coated filtration membranes will purify polluted water and separate carbon dioxide from air. Coatings will protect and reduce spoilage of food. Future improvements in corrosion protection will increase the longevity of expensive assets and reduce pollution resulting from the failure of pipes carrying oil and other chemical feedstocks. This article will detail the progress made to date and the future challenges in coatings science.
Past Accomplishments
The growth of environmentally friendly coatings technologies such as waterborne, powder and high solids has progressed steadily to the point where nearly 75% of global coatings sales are derived from these technologies. This trend is projected to continue into the future (Figure 1).4
Environmental improvements enabled by coatings technology can be grouped into several main categories:
- Coating solvent content reduction;
- Elimination of toxic raw materials;
- Reducing cure energy/temperature;
- Simplified paint processes;
- Application efficiency;
- Coating-enabled asset lifetime increase (corrosion, photo-oxidation);
- Coating-enabled energy savings (lightweight substrates, drag reduction, heat dissipation, heat reflection).
Current Issues/Opportunities
While the coatings technical community has much to be proud of, we should not be complacent because much remains to be done. Continued issues with air pollution in China have prompted the Chinese government to impose a VOC tax on all coatings sold in China that have more than 420 g/L VOC.5 Hexavalent chromium remains a preferred method of corrosion protection in aerospace and industrial coatings because no better alternative has been found. Concern about cobalt driers in alkyd paints has prompted a search for more environmentally benign alternatives.6 The use of copper salts in antifouling coatings for ships is a matter of current debate7 in the wake of a ban of tributyl tin use for this application several decades ago.
Bisphenol A (BPA) has been banned in coatings for baby food containers, and more recently in France in beverage and food containers.8 BPA has recently been added to California’s Proposition 65 list of substances known to cause reproductive hazards.9 Whether or not BPA is actually hazardous at the levels found in can coatings is becoming a moot point. This is causing the can coating industry to wonder “what’s next” in terms of allowable coating materials and will undoubtedly continue to drive change in coatings chemistry. N-methyl pyrrolidone (NMP) solvent has been removed from coatings to certain automotive customers.10 Isothiazolone preservatives are under scrutiny in Europe.11 The use of nonyl phenol-based surfactants is being phased out.12 Even TiO2 safety has been called into question.13 These and other safety concerns will continue to drive change in the coatings industry. Regulatory issues are not the only change drivers in our industry, however.
Drivers of Change in the Coatings Industry
Government Regulation
Regulations designed to improve product safety and reduce pollution from coatings are pervasive globally, as discussed in the last section. Other types of regulation designed to reduce the environmental impact of coated objects can also affect coating chemistries. One example is the Corporate Average Fuel Economy (CAFE) standard in the United States, which requires the average fuel efficiency for cars and light trucks to be 54 miles per gallon by 2027.14 This regulation will drive large changes in the transportation sector in the coming years. The change has already begun with the increased use of lightweight substrates like aluminum, magnesium, high-strength steel and composites in vehicles.
Increased electrification will improve the average fuel efficiency and reduce the carbon footprint of the global vehicle fleet. This trend is already visible with many new cars having start/stop systems as standard equipment, and plug-in electric vehicles becoming more prevalent. The electrification trend is expected to continue into the future. In fact, consumers in China may be early adopters of the technology due to widespread concern about air pollution and government subsidies of the technology.15 Continued improvement in battery technology will accelerate the change to electric vehicles as “range anxiety,” perceived safety and charging infrastructure issues are addressed. At the Paris climate conference in December 2015, 195 countries adopted the first-ever universal, legally binding global climate deal.16 Whether or not rising temperatures are caused by human activity, there appears to be growing political will to take action.
Coatings can have a great impact on these global needs. Lightweight substrates will require new ways to mitigate corrosion. Vehicles will require new joining methods for composites, and dissimilar metals will need to be isolated to avoid galvanic corrosion. The corrosion requirements for new fasteners will be more difficult to achieve. Even carbon fiber-reinforced composites can cause galvanic corrosion if they are not isolated from other metals.17 The coating of advanced high-strength steel (which enables light weighting by down-gauging the metal) presents corrosion challenges as a result of the silicon found in some alloys. Silicon dioxide formed during welding can interfere with coating deposition by electrodeposition (Figure 2).
Familiar corrosion mitigation methods may not be optimal for new metals like aluminum and magnesium. Sound control in airplanes and cars requires new damping solutions as the resonant frequencies of these new substrates change.18
Cost
Even as coatings technology continues to advance, the “cost of painting” remains a key consideration for coatings customers. The cost of the paint is one aspect of total cost, but application costs, surface preparation, the energy required to cure thermoset coatings and transfer efficiency are all important aspects of the total cost of a coated object. TiO2 is an important raw material in many paints, plastics and cosmetics because of its high refractive index and unique ability to provide opacity to these products. Price fluctuations for this and other raw materials have caused much angst in the coatings community over the last several years. Supplier relationships and development are key to the maintenance of a robust supply chain. These interactions have resulted in a more stable price structure for TiO2 in recent years.
Looking to the future, another example of a potentially important raw material is graphene. This material has received much attention in the literature and popular press.19 It could conceivably be useful in coatings and other materials as a pigment or as an electrically conductive material for diverse applications such as static dissipation in tires or conductive inks. In order for these applications to come to fruition, the cost of the material must be reduced from current levels. New synthesis methods show promise for the eventual reduction in the cost of this potentially important raw material.20 Time will tell if these predictions come true, but as coatings technologists, we must take an active role in supply chain security and the development of useful new materials.
Another important aspect of total cost is the cost of the coating process. “Compact process” automotive painting is now spreading through the industry because of energy savings resulting from the elimination of one of three main curing ovens in a typical automotive paint line, along with some of the associated sanding operations. This has been done without sacrificing coating durability or physical properties. Up to 30% energy savings (relative to the traditional process) has been realized with this new process. Additionally, improvements in plant productivity, greenhouse gas emissions and VOC emissions have been realized (Figure 3).21
Performance/Functionality
Increase in the performance of coatings is at the forefront of technologist’s minds as they develop new products, and performance certainly can be a driver of change in our industry. Performance characteristics such as corrosion resistance, damage tolerance (scratch, stone chip), color, photo-oxidation resistance and smoothness/appearance have been and will continue to be the focus of research. Several novel approaches have been developed recently.
Traditional requirements for coatings have involved protection and decoration of objects. Decoration involves color control and new ways to generate long-lasting color. Nanopigments provide deep, transparent colors. Encapsulation of these pigments has enabled their use in waterborne and powder coatings without agglomeration, allowing vibrant colors in these historically demanding applications.22 Crystalline colloidal arrays of polymer spheres can provide vibrant colors that change with viewing angle.23 These materials can be useful in security applications, as sensors, and as colorants. Certain dyes can be incorporated into coatings to indicate impact damage to a coated part.24
The scratch resistance of automotive clearcoats has been improved by reinforcement with nanoparticles.25 Infrared reflective coatings keep the interiors of buildings and planes cooler. Omniphobic coatings now protect cell phone screens and make them easy to clean. Sound-absorbing coatings make cars quieter. Antifouling coatings free of heavy metals are available to keep ship hulls clean and save on energy costs (Figure 4). Many other performance attributes are being improved every day in coatings labs around the world.
Future Opportunities
If we look to the future, there are several trends that could be addressed by the coatings industry in markets that most would consider nontraditional. Waste reduction and cost are driving many manufacturers to consider additive manufacturing methods. Because of this, printable electrically conductive inks that are used in an “additive” manner as opposed to subtractive etching techniques are being used in RFID tags, mobile device antennas, flexible heating devices and many other everyday items. Future use in photovoltaic and OLED devices is predicted.26 We have developed carbon-based inks with good conductivity (Figure 5). Graphene nanoplatelets (GNPs) have proven useful in this application. Scalability and cost will determine the viability of this approach.
Batteries are composed mainly of coated metal. Battery actives are typically metal oxides. The coatings industry is experienced in the dispersion of metal oxides. We know how to control defects and improve adhesion to metal surfaces. These competencies will impact the issues that battery manufacturers face today. One example of an issue that the battery industry faces is the possible need to eliminate NMP from battery-active coatings for regulatory and cost reasons. There is also industry interest in waterborne coatings for batteries. The coatings industry has experience with such matters. The use of graphene in battery coatings may also be beneficial. As seen in Figure 6, GNP-based battery cathodes can be formulated with better capacity retention relative to those made of conventional carbon.
Corrosion on pipes carrying petroleum and other chemicals, and on infrastructure in severe environments like offshore oil rigs and deep wells, could be improved as failure of these assets cannot be tolerated. Vehicle underbody corrosion has been a problem at times. As mentioned previously, coatings with improved corrosion resistance are needed in order to enable the use of thinner metal and thus allow light weighting. This is a challenge for the whole industry. Improved performance is possible as demonstrated by new zinc-rich primer corrosion results (Figure 7).
Summary
There is indeed much to be proud of as a coatings technology community. Much progress has been made toward our common goal of providing future generations with a clean and safe world to live in. Much remains to be done. New and old challenges require new and better solutions. The collective innovations that we strive so hard to develop are making a difference. In order to affect true change, we must develop products that touch on several of the change drivers that have been discussed, including regulatory issues, total cost of painting and performance. New products that improve at least two of these drivers have a high probability of success.
References
1 American Coatings Association. Facts about the Paint & Coatings Industry: Addressing Environmental Issues Proactively; http://www.paint.org/about-our-industry/environmental-footprint/ (accessed 1/8/2016).
2 Paintcare Home Page. http://www.paintcare.org/ (accessed Dec 23, 2015).
3 PPG IdeaScapes Homepage. http://www.ppgideascapes.com/Metal-Coatings/Products/Coil Coatings/Building-Roof-Wall-Panels/Duranar-Vari-Cool-Coatings.aspx (accessed Dec 23, 2015).
4 Srinivasa R. Global Markets and Advanced Technologies for Paints and Coatings; BCC Research: Wellesley, MA, 2014; p 7.
5 China Slaps VOC Tax on Coatings. http://www.paintsquare.com/news/?fuseaction=view&id=12741 (accessed Dec 30, 2015).
6 CEPE information bulletin about cobalt driers. http://www.vslf.ch/download.php?file_id=695&download=true (accessed Dec 30, 2015).
7 Lydecker, R. Is Copper Bottom Paint Sinking?. http://www.boatus.com/magazine/2012/february/copper.asp (accessed Dec 30, 2015).
8 France Overturns Ban on BPA in Export Products. http://www.euractiv.com/sections/health-consumers/france-overturns-ban-bpa-products-export-317809 (accessed Dec 30, 2015).
9 Bisphenol A Listed as Known to the State of California to Cause Reproductive Toxicity, Effective May 11, 2015. http://oehha.ca.gov/prop65/CRNR_notices/list_changes/051115listBPA.html (accessed Dec 31, 2015).
10Opinion on N-Methyl-2-pyrrolidone (NMP). http://ec.europa.eu/health/scientific_committees/consumer_safety/docs/sccs_o_050.pdf (accessed Dec 31, 2015).
11CEPE Guidance on Labelling Decorative Paint with Skin Sensitizing Biocides. http://sveff.se/files/2015/04/CEPE-guidance-on-labelling-with-biocides-skin-sensitizers-final-28-Oct-14.pdf (accessed Jan 1, 2016).
12EU directive restricting the use of nonylphenol ethoxylate and nonylphenol products. http://www.tfl.com/web/files/Statement_NPE-surfactants.pdf (accessed Jan 3, 2016).
13Titanium Dioxide Classified as Possibly Carcinogenic to Humans. https://www.ccohs.ca/headlines/text186.html (accessed Jan 3, 2016).
14National Highway Traffic Safety Administration webpage. http://www.nhtsa.gov/fuel-economy (accessed Jan 3, 2016).
15Moore, T. CMU Study Finds Chinese Consumers May Adopt Electric Vehicles First, Impacting Auto Market. http://www.cmu.edu/news/stories/archives/2015/february/china-and-electric-vehicles.html (accessed Jan 3, 2016).
16European Commission Webpage. http://ec.europa.eu/clima/policies/international/negotiations/future/index_en.htm (accessed Jan 3, 2016).
17Yari, M. Galvanic Corrosion of Metals Connected to Carbon Fiber Reinforced Polymers, https://www.corrosionpedia.com/2/1556/corrosion/galvanic-corrosion-of-metals-connected-to-carbon-fiber-reinforced-polymers (accessed Jan 3, 2016).
18Pelinescu, I. LASD Coatings Optimized for Damping of Aluminum Intensive Vehicle Bodies, https://www.lbcg.com/media/downloads/events/471/day-3-1-50-ion-pelinescu-ppg-industries-inc.8137.pdf (accessed Jan 3, 2016).
19Graphene. https://en.wikipedia.org/wiki/Graphene (accessed Jan 3, 2016).
20Vanier, N. Low Cost Plasma Synthesis of Graphene Nanoplatelets from Methane, http://www.idtechex.com/events/presentations/low-cost-plasma-synthesis-of-graphene-nanoplatelets-from-methane-004568.asp (accessed Jan 3, 2016).
21Furar, J. Coatings and Process Development for Reduced Energy Automotive OEM Manufacturing, http://energy.gov/sites/prod/files/2015/06/f22/R3-DE-EE0005777%20PPG%20AMO-PR-2015.pdf (accessed Jan 3, 2016).
22Fenn, D. R.; Faler, D. L.; Anderson, L.; Roper, T. Aqueous Dispersions of Microgel Encapsulated Particles Utilizing Hyperbranched Acrylic Polymers. U.S. Patent 9040621, May 26, 2015.
23Vanier, N. R.; Donnelly, J.T.; Xu, X. Highly Reflective Crystalline Colloidal Arrays with Radiation Absorbing Particles. U.S. Patent 2015/0205010 Al, Jul 23, 2015.
24Chopra, A.; Deng, J.; Hickenboth, C.R.; Peffer, R.M. Mechanochromic Coating Composition. U.S. Patent 8,815,771, Aug 26, 2014.
25Schmeltzer, R.; Donaldson, S.; Olson, K. C.: Olson, K. G.; Schwendeman, J. E.; Simpson, D. E.; Williams, F. C. Coating composition comprising an alkoxysilane, a polysiloxane, and a plurality of particles. US Patent 8,563,648, Oct 22, 2013.
26Nir, M. M.; Zamir, D.; Haymov, I.; Ben-Asher, L.; Cohen, O.; Faulkner, B.; de la Vega, F. “Electrically Conductive Inks for Inkjet Printing” in: Magdassi, S. (Ed.) “The Chemistry of Inkjet Inks”, World Scientific, 2010. Nir, M. M.; Zamir, D.; Haymov, I.; Ben-Asher, L.; Cohen, O.; Faulkner, B.; de la Vega, F. Electrically Conductive Inks for Inkjet Printing. in The Chemistry of Inkjet Inks; Magdassi, S. (Ed.) World Scientific, 2010.
This paper was presented at the 2016 Waterborne Symposium in New Orleans.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!





