Metal Adhesion and Corrosion Resistance of Coatings

Corrosion has been defined as an interaction of a material with its environment that results in an irreversible degradation of the material.1 Although organic coatings are often used to prevent the surface of a material from coming into contact with the corrosive environment, compromises in the formulation of many water-based coatings to improve adhesion can reduce their effectiveness at abating corrosion. Recent work with hybrid styrene acrylic polymers containing hydrophobic resin has shown that an improved balance of properties is possible compared to conventional acrylic or styrene acrylic polymers.
The reaction that takes place during corrosion of steel is caused by the creation of an electrochemical cell. This electrochemical cell needs four components to function – an anode, a cathode, a connection between the two and an electrolyte. The surface of steel is rather heterogeneous and has sites that can be anodic or cathodic, and the connection between them is provided by the steel itself. If the steel is exposed to an electrolyte like seawater, then all four necessary components of the cell are present and the corrosion on the surface of the steel will begin.
If an organic coating is applied to steel then the electrolyte (with its water, oxygen and ions) can theoretically be kept separate from the steel. Unfortunately, water and oxygen can penetrate most organic coatings fairly easily.3,5 The ions cannot penetrate the organic coating as easily; most organic coatings are very good barriers to ions.3 There must be channels through the coating to the surface that will conduct the ions for corrosion to start on coated steel. Some authors3,4 have proposed that liquid water in contact with the coating can create these conductive channels that allow the transport of the ions to the surface.
The proposed mechanism5 for creation of the conductive channels (Figure 1) begins with water uptake of the coating film. This water uptake does not occur evenly across the coating surface, however. Water is first absorbed into hydrophilic pockets that are composed of low-molecular-weight polymer units, surfactants or salts from the polymer or coating formulation. These pockets are created between polymer structure units as the film dries because the free hydrophilic ingredients migrate into areas of residual water during dry. When the dry film is later exposed to liquid water, the hydrophilic pockets can absorb a significant amount of water, with some authors estimating 45% -75% by weight3,5 as opposed to just 5% by weight for the polymer units. As they absorb water these pockets swell along the boundaries of the polymer units, and eventually link up to form a pathway through the coating. This pathway allows ions to pass freely through the coating. The ions, along with the water and oxygen, can initiate the corrosive reactions at the surface of the steel.
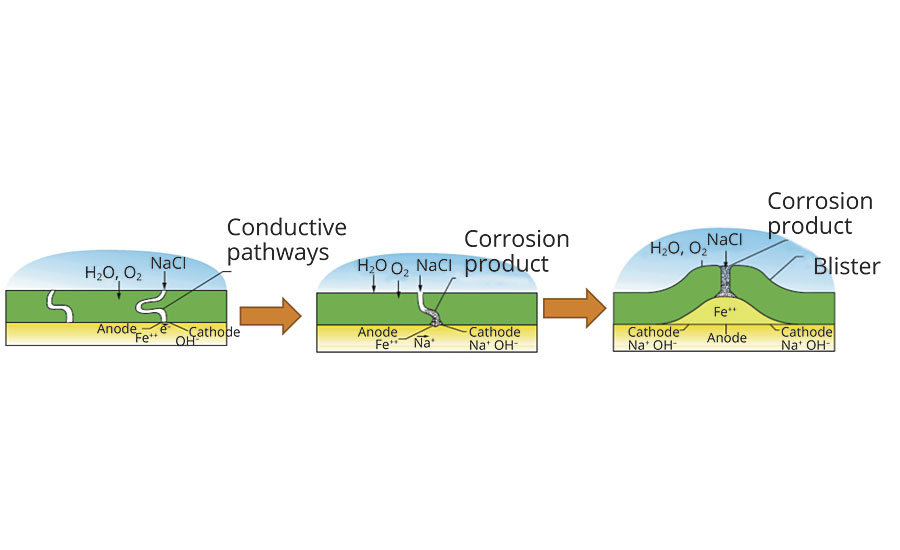
FIGURE 1 » Schematic of corrosion in coated steel (from Nguyen, T.; Hubbard, J.B.; Pommersheim, J.M. 19965 )..
Once the ions have reached the surface through conductive pathways, the passivating layer of metal oxide at the surface is degraded (a process accelerated by the chloride ion2) and the anode is created at the base of the conductive pathway. The iron is oxidized at the anode, creating soluble ferrous ion and releasing a pair of electrons that travel through the steel to the cathode created on the surface immediately adjacent to the anode. At the cathode the dissolved oxygen is reduced to hydroxyls that are counterbalanced with the sodium ions from the electrolyte.
The hydroxyls have a significant effect on both the coating and the progress of corrosion. The sodium hydroxide raises the pH of the electrolyte under the coating – some authors estimate that the pH of that solution can be as high as 11.7 If the coating is sensitive to alkali, then the high pH solution can soften it and may cause it to disbond from the surface. The hydroxyls may also change the oxide layer of the steel surface to make disbondment easier.7 In addition, the sodium hydroxide is hygroscopic and it raises the ionic strength of the solution. If the ionic strength is greater than the ionic strength of the solution outside the coating, then osmotic forces pull more water through the coating to balance the ionic strength between the inside and outside of the coating. If the osmotic force is greater than the adhesion of the coating to the substrate, then the osmotic force pushes the coating off the surface and contributes to the formation of the blister. These blisters eventually grow and coalesce together, giving complete loss of coating adhesion.
For this mechanism, there are several ways that a water-based coating might contribute to corrosion resistance. If the total amount of free hydrophilic species in a coating – from both the polymer and the coating formulation itself – can be kept low, then the number and size of the hydrophilic pockets that grow to form the conductive pathways can also be kept low. In the coating formulation, this is done with the use of hydrophobic dispersants and thickeners. Polymer design can also help minimize formation of hydrophilic pockets. If the surfactant level is reduced, and stabilizing acid functional monomers or polymerizable surfactants are attached to the outside of the polymer particles then there is less migration to the hydrophilic pockets formed as the film dries. In addition, acid-functional monomer and phosphate-functional polymerizable surfactants can significantly improve adhesion to the metal. Theoretically, improved adhesion can resist the osmotic forces pulling the coating from the surface, however if too much acid or phosphate surfactant is used, the coating is more hydrophilic and it can have worse corrosion.
One way to make the high acid polymers less hydrophilic while maintaining good adhesion is to use a hydrophobic monomer like styrene in the polymerization. In order to improve corrosion resistance, a relatively high level of styrene must be used, however, and at that level, the styrene can detract from the exterior durability. For these reasons, the balance of very good corrosion resistance, good QUV gloss retention and very good adhesion is hard to achieve for water-based DTM paints.
A novel hybrid styrene acrylic, RayKote™ 2020, which is polymerized with a low level of hydrophobic resin, has been developed to improve the adhesion and corrosion properties of DTM coatings. The level of acid-functional monomer used was chosen to give very good stability and adhesion. The stabilizing surfactant was a combination of phosphate reactive and conventional surfactants, but the total surfactant level was reduced to the minimum level that gave good polymer stability. Because of the hydrophobicity of the resin used to form the hybrid, the level of styrene used in the polymer could be kept low to maintain exterior durability.
Materials and Methods
In order to test if the improved corrosion resistance was a result of improved adhesion, an analogue of the hybrid styrene acrylic was prepared without the hydrophobic resin (Variant 1). Formula 1, a gloss DTM paint at 39% volume solids, 15.5% PVC and <100 g/L VOC, was prepared (Table 1). This paint was made with a hydrophobic dispersant and HEUR thickener.
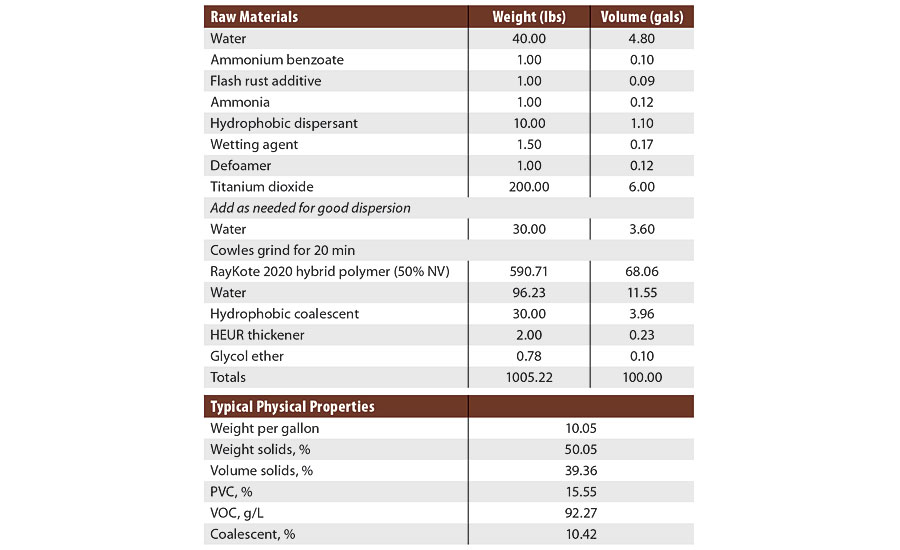
TABLE 1 » Experimental gloss DTM paint (Formula 1).
Dry and wet adhesion were tested using the ASTM D3359-97 “Standard Test Methods for Measuring Adhesion by Tape Test”, Test Method B. Test substrates were Q-Panel R36 dull matte finish cold rolled steel, Q-Panel S36 ground one side cold rolled steel, #32172 galvanized test panels from ACT, Q-Panel R-46-I dull matte Bonderite-treated cold rolled steel, and Q-Panel A-36 untreated aluminum. Cold rolled steel and galvanized panels were cleaned with MEK, and each sample was drawn down using a 3 mil bird bar and aged for one week. Dry and wet adhesion were tested using the knife peel and cross hatch tape pull method and blistering, if any, was observed.
QUV-A resistance was tested following ASTM G154-06 Cycle 1 in a Q-Panel QUV Accelerated Weathering Tester. Paints were drawn down on treated aluminum Q-panels using a 5 mil path depth applicator blade and aged for one week. Panels were exposed to 300 hrs of accelerated weathering with QUV-A 340 bulbs and cycling 8 hrs of light followed by 4 hrs of dark and condensation. The initial and final 60° gloss of the panel was measured using a Rhopoint Novogloss glossmeter.
Corrosion resistance was evaluated using ASTM B117-16 protocol for salt fog/spray. Samples were drawn down using a 3-mil wet film thickness Bird blade on cleaned Q-Panel R36 matte cold rolled steel. Panels were exposed in a Q-Fog SSP-600 cyclic corrosion chamber using 5% NaCl solution for 96 hrs. Field rust, undercut at scribe and blistering were evaluated for each panel.
Electrochemical impedance testing was run on coating drawdowns at 3 mils wet film thickness using a Bird blade on Q-Panel R36 dull matte finish cold rolled steel panels. Coated panels were exposed to 5% NaCl solution for 6 days. Electrical impedance was tested at 0.1 Hertz at 24-hr intervals. Testing was done by NEI Corporation of Somerset, NJ.
Results: Adhesion and Corrosion Testing
Adhesion and corrosion resistance test results for DTM paints made with the hybrid styrene acrylic with hydrophobic resin and the styrene acrylic without the hydrophobic resin are displayed in Table 2. Both polymers had very good adhesion to steel substrates, which may indicate that the acid and phosphate surfactant level in the polymers was high enough to provide good adhesion. Although polymers with better adhesion might be expected to have better corrosion resistance and blistering (because the adhesive forces to the substrate would balance the osmotic forces that drive the growth of the blisters), if the acid or phosphate surfactant makes the boundary areas around the polymer particles more hydrophilic, this might increase the number of conductive pathways through the film. In ASTM B117-16 salt fog testing (Figure 2), the corrosion resistance was significantly better for the hybrid styrene acrylic with hydrophobic resin. This similarity in adhesion and difference in corrosion resistance may indicate that at the same acid and phosphate surfactant level, the effect of the hydrophobic resin was to reduce the number of conductive pathways through the film, which improved the corrosion resistance.

TABLE 2 » Adhesion and salt fog testing.
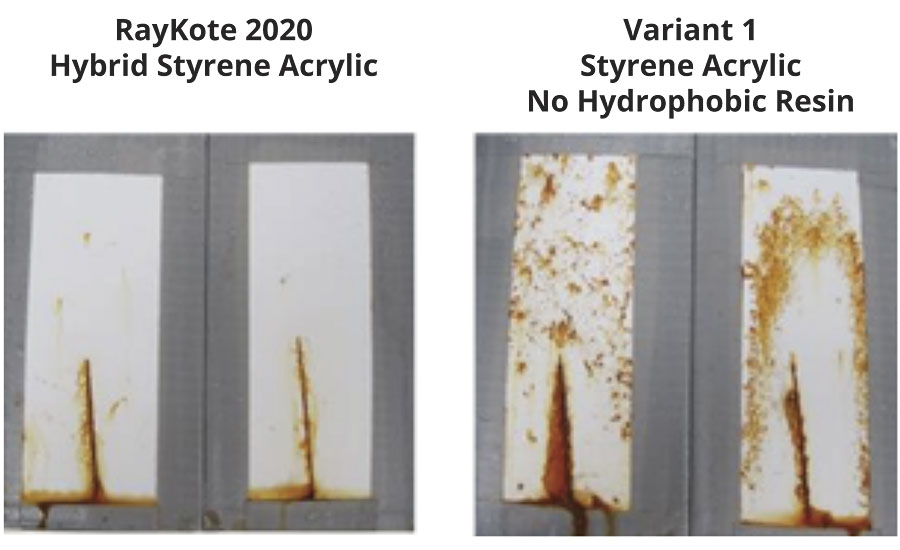
FIGURE 2 » Cold rolled steel panels after 96 hrs of ASTM B117-16 salt fog.
Results: Electrochemical Impedance Testing
Water uptake of a film can be tracked over time using electrochemical impedance testing because water changes the dielectric properties of the film. The water uptake changes the coating capacitance, which increases with time until the film becomes fully saturated with water and the capacitance levels off.6 The paint based on hybrid styrene acrylic had significantly higher impedance than the variant without the hydrophobic polymer (Figure 3). The higher impedance of the hybrid styrene acrylic compared to Variant 1 polymer may indicate that the hydrophobic resin in the hybrid polymer is improving corrosion by reducing the number of conductive pathways through the film.
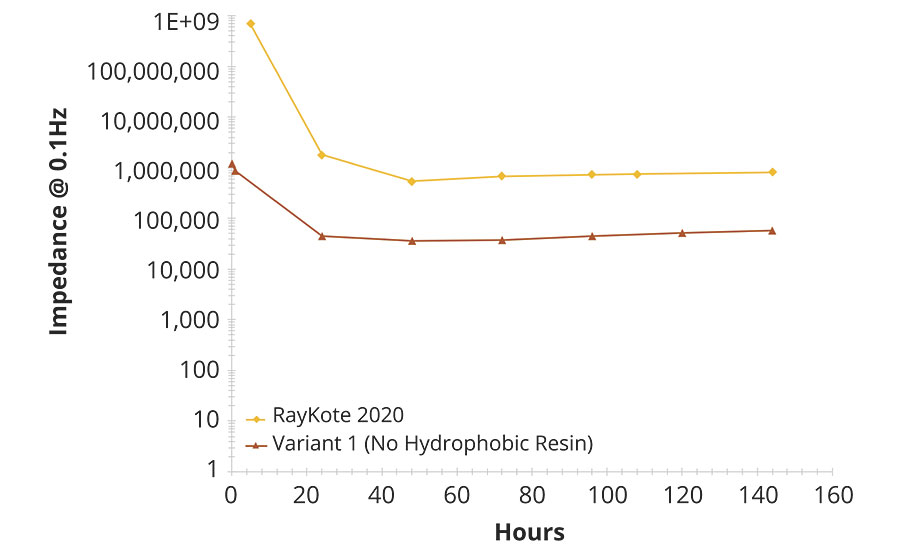
FIGURE 3 » EIS testing at 0.1 Hz: hybrid styrene acrylic vs variant 1 without hydrophobic resin.
Comparison to Commercial Low-VOC DTM Polymers
The hybrid styrene acrylic was compared to two competitive low-VOC DTM polymers. The competitive polymers were a self-crosslinking styrene acrylic with a Tg of 17 °C (Comp 1) and an all-acrylic without self crosslinking and a Tg of 27 °C (Comp 2). The paints were 34.6% volume solids, 17.7% PVC and formulated to be <100 g/L VOC.
Results: Adhesion and Corrosion of Commercial Low-VOC DTM Polymers
The hybrid styrene acrylic with hydrophobic resin was compared to commercial low-VOC polymers designed for DTM applications (Table 3). In this test, the hybrid styrene acrylic had better adhesion to the different metal substrates than the commercial low-VOC DTM polymers. The corrosion resistance of the hybrid styrene acrylic was much better than one of the commercial DTM polymers and equal to the other (Figure 4). All three polymers had very good QUV-A gloss retention after 300 hrs of testing.
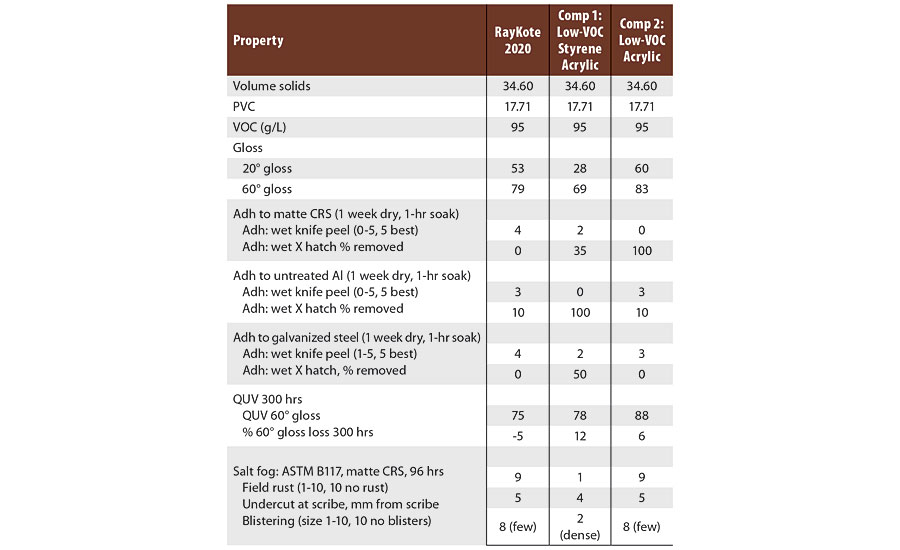
TABLE 3 » Adhesion, QUV resistance and salt fog testing of commercial low-VOC DTM polymers.

FIGURE 4 » Corrosion resistance after 96 hrs of ASTM B117 salt fog testing.
The hydrophobic resin in the hybrid styrene acrylic contributed to a different balance of properties than the commercial low-VOC DTM polymers. The hydrophobic polymer allowed the use of acid-functional monomers at a level that gave superior wet adhesion to metal but, possibly because of the additional hydrophobicity, the acid did not detract from the corrosion resistance. The hydrophobic resin in the hybrid styrene acrylic gives enough hydrophobicity that only a low level of styrene is required for good corrosion resistance, and this gave very good QUV gloss retention.
Conclusion
Using a low level of a hydrophobic polymer during the polymerization of a styrene acrylic emulsion polymer significantly improved the corrosion resistance compared to a similar styrene acrylic without the hydrophobic polymer. The wet adhesion of paints made with both polymers was excellent on CRS, but the corrosion resistance for the hybrid polymer was significantly better than the polymer without hydrophobic resin. This suggests that film hydrophobicity may be contributing to the improved corrosion resistance. Electrochemical impedance testing showed that the conductive pathways developed to a lesser extent in the polymer with the hydrophobic resin, suggesting that the number or the segregation of hydrophilic pockets in the film may account for the difference that was found in the corrosion resistance of the polymers.
The hybrid polymer was also found to have an improved balance of properties compared to two commercial low-VOC emulsion polymers designed for the DTM market. The improved adhesion, good corrosion resistance and good QUV gloss retention show that the hydrophobic resin in the hybrid polymer can be used to reduce the effects of high acid on corrosion and of styrene on QUV gloss retention.
References
1 Popoola, A.; Olorunniwo, O.E.; Ige, O.O. Corrosion Resistance Through the Application of Anti-Corrosion Coatings, Developments in Corrosion Protection, Dr. M. Aliofkhazraei (Ed.), InTech, DOI: 10.5772/57420, (2014).
2 Thomas, J.G. (n.d.). The Electrochemistry of Corrosion (G. Hinds, Ed.). Retrieved February 17, 2018, from https://www.scribd.com/document/211839741/The-Electrochemistry-of-Corrosion-With-Figures.
3 Corti, H.; Fernandez-Prini, R.; Gomez, D. Protective Organic Coatings: Membrane Properties and Performance. Progress in Organic Coatings, 10, 5-33, (1982).
4 Kendig, M.W.; Leidheiser, H., Jr. The Electrical Properties of Protective Polymer Coatings as Related to Corrosion of the Substrate. Journal of the Electrochemical Society,123(7), 982-989, (1976).
5 Nguyen, T.; Hubbard, J.B.; Pommersheim, J.M. Unified Model for the Degradation of Organic Coatings on Steel in a Neutral Electrolyte. Journal of Coatings Technology, 68(855), 45-56, (1996).
6 Moreno, C.; Hernández, S.; Santana, J.J.; González-Guzmán, J.; Souto, R.M.; González, S. Characterization of Water Uptake by Organic Coatings Used for the Corrosion Protection of Steel as Determined from Capacitance Measurements. International Journal of Electrochemical Science, 7, 8444-8457, (2012).
7 Ritter, J.J.; Kruger, J. A New Technique to Study Corrosion Mechanisms Under Organic Coatings. In Passive Films, Surface Structure and Stress Corrosion and Crevice Corrosion Susceptibility (pp. 1-30). Washington, DC: U.S. Department Of Commerce, (1984).
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!







