New Generation of Alkyl Phenol-Free Nonionic Surfactants for Emulsion Polymerization
About 10 years ago, Henkel Corporation, now Cognis Corporation, developed a family of nonionic green surfactants for emulsion polymerization called the Disponil A Series. They were developed as alternatives to traditionally used APEs and synthetic Alcohol Ethoxylates (synthetic AEs).
Last year, an upgrade of European toxicological regulations resulted in a new safety label for the low-molecular-weight products in the Disponil A series. This additional eco-toxicological labeling prompted Cognis to develop a second generation of nonionic surfactants called Disponil AFXs. Although the Disponil A products will continue to be manufactured, it is important to note the added benefits of the new series. Disponil AFX emulsifiers have better performance, cost effectiveness and lower toxicity than many other commercial nonionic surfactants. Similar to the first generation, the introduction of these new emulsifiers responded to the emulsion polymerization industry demands for ecologically acceptable and effective nonionic emulsifiers.
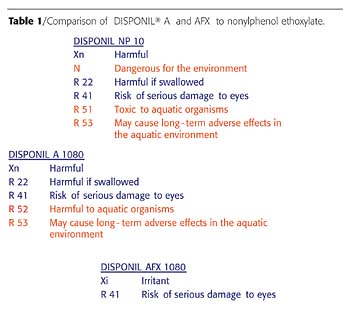
European Eco-Toxicological Classification
The 2002 European eco-toxicological upgrade dictated the use of new safety labeling. The low ethoxylates in the Disponil A series, those bearing 10 moles of ethylene oxide (EO) or less, were subjected to additional labeling. Table 1 compares the eco-toxicological labeling for three low-molecular-weight products bearing the same ethoxylation level of 10 moles of EO.While nonyl phenol 10-EO (NP-10) exhibits most of the negative labeling, Disponil A 1080 shows the R52 and R53 negative phrases added last year in Europe. In contrast, Disponil AFX 1080 is free of negative environmental danger labeling, making it more acceptable.
One of the significant benefits of low-toxicity products such as the Disponil AFXs is that they can be used in "green label" formulations.
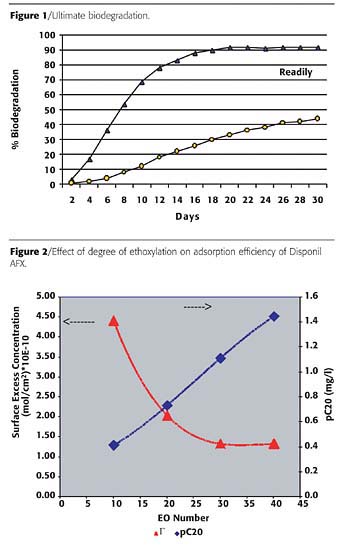
Biodegradation
Surfactant biodegradation has been extensively discussed elsewhere.1-3 The ultimate biodegradation or mineralization of Disponil AFX emulsifiers was determined according to OECD 301F by measuring the loss of dissolved organic carbon (DOC). The AFX surfactants passed the ‘readily biodegradation' test. By definition, a readily biodegradable substance should biodegrade at least 60% during the first 10 days of the test to an extent of at least 70% in 28 days.Figure 1 shows examples of two different biodegradation pathways. Readily biodegradable surfactants show high biodegradation rate and extent of biodegradation. In contrast, substances that are difficult to biodegrade show a very slow initial rate of biodegradation as well as a low percentage or extent of biodegradation. In addition, some of these chemicals tend to linger in the environment for longer periods of time, in some instances degrading to small molecular species with higher toxicity.
Examples of other commercially available, readily biodegradable surfactants are many sulfates, sulfosuccinates, and sulfonates such as SLS, DOS and LSDBS. Some good questions that arose after examining the readily biodegradation pathways are: "What is the impact of having readily biodegradable surfactant/s in the formulations?" Or, "How would the latexes or the dry coatings be affected by the readily biodegradable surfactants in the formulations?" So far, aging studies of coatings made with other readily biodegradable surfactants have not shown specific deterioration associated with these emulsifiers. Thus, until long-term aging study results are available, it is logical to assume that as long as the finished latex and coating products are initially well protected against bacteria and mold growth, the use of these biodegradable surfactants would not negatively impact their performance or appearance.
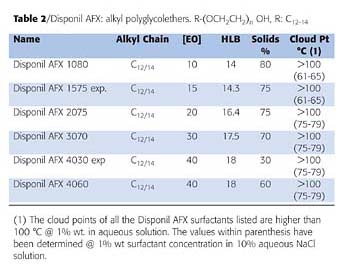
Surfactant Composition and Properties
Disponil AFXs are clear, high-solid liquids at room temperature, exhibiting excellent water miscibility and performance in emulsion polymerization.
Table 2 lists the products, composition and properties. The hydrophobe in the Disponil AFX products is mostly made from natural renewable resources. This portion of the molecule is based on C12-14 fatty alcohols consisting of primary and secondary alcohol isomers.
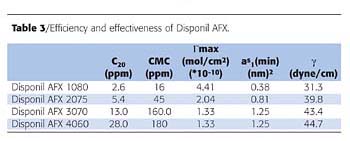
Structure/Property Relationships of Disponil AFX Surfactants
Adsorption of Disponil AFX surfactants at the air/water interface was investigated through surface tension measurements at 25 ºC using the Wilhelmy plate method and a Krüss surface tensiometer. Dynamic interfacial tensions were measured using the pending drop method and the Tracker drop tensiometer from IT concept. The results are summarized in Tables 3-5 and Figures 2-5. The surface excess concentration Gmax defines the effectiveness of adsorption of the surfactants at either air/water or water/monomer interfaces. Gmax was calculated from the Gibbs Equation according to:
1 dg
G = --- * ---
RT d(lnC)
where G (mol/cm2) refers to the surface excess concentration, g is the interfacial tension for either air/water or water/monomer systems. The surface excess is obtained from the slope of g vs lnC plot. The area (as) occupied by the molecule at the interface is inversely proportional to G. The calculation for as (m2/molecule) is shown as follows:
as = 1016 / NA G
The efficiency of the surfactant refers to its ability to reduce surface and interfacial tensions. The bulk concentration of surfactant necessary to reduce the surface tension by 20 dynes/cm, C20, is a good measure of the efficiency of the surfactant.
Efficiency and Effectiveness of Disponil AFXs at Air/Water Interface
Table 3 presents the efficiency and effectiveness of the AFX surfactants. As expected, the surface activity of AFX is greater for the surfactants that contain a lower EO unit number.
The minimum surface tension is g. CMC is the critical bulk concentration needed for micelle formation. C20 represents the bulk concentration of surfactant required to reduce the surface tension by 20 dynes/cm. The lower the number, the more efficient is the surfactant. At the C20, 85% of the interface is covered with surfactants. The maximum interfacial concentration is represented by Gmax, while as1 (min) is the minimum area that a molecule of surfactant occupies at the interface.
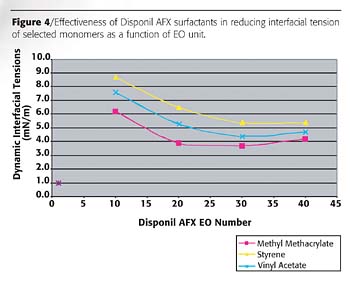
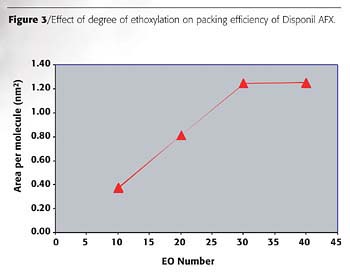
Figure 3 shows the change in the packing of Disponil AFX surfactants as EO content increases. The area occupied by the surfactants is a critical parameter in predicting the effectiveness of adsorption and the stabilizing effect of the surfactant on emulsion films. Small values of asmin lead to tightly packed and coherent interfacial films. The area occupied by the hydrated hydrophilic groups mainly determines asmin. The area occupied by the surfactant molecules increases as EO content increases and reaches a plateau at 30 EO. The Disponil AFX 1080 at the air/water interface shows a closer packing than AFX 4060, which is highly hydrated.
At the water/monomer interface, the interactions between the surfactant hydrophobic group and the monomer affect the surface area occupied by the surfactants.
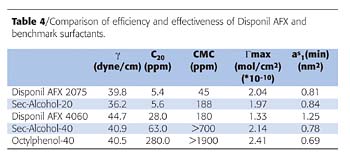
Effect of Surfactant Hydrophobic Group on Efficiency and Effectiveness of Adsorption
Table 4 compares the effect of hydrophobic groups on the efficiency and effectiveness of adsorption of Disponil AFX and benchmark surfactants. In other words, we compared the effect of the chemical structure of Disponil AFX, secondary alcohols and octyl phenol. At any EO content, the AFXs are more efficient surfactants than the secondary alcohols and octyl phenols, as shown by the minimum concentrations at C20 and CMC observed for Disponil AFXs. It is also worth noting that equilibrium could not be maintained at CMC for the octyl phenol-40 and sec-alcohol-40 samples.
As the number of EO units increases, the effect of hydrophobic groups are more evident. Table 4 shows the effect of hydrophobic groups on adsorption efficiency. With 20 EO units, Disponil AFX 2075 behaves similarly to sec-alcohol-20. As the EO unit number increases, a difference is observed in the packing of the surfactants at the interface. The phenyl group in octyl phenol-40 seems to force the molecule to coil and lie perpendicular to the surface, while Disponil AFX 4060 lies flat on the interface.
Effectiveness of Disponil AFX at Water/Monomer Interfaces
Figure 4 presents both the effect of chemical structure (the hydrophilic group of the surfactants and the chemical structure of the monomers) on liquid/liquid interfacial tensions (IFT) for the series of AFX surfactants. The monomers investigated are methyl methacrylate, vinyl acetate and styrene. In all cases, the interfacial tension decreases as the EO unit number of the surfactants (and HLB) increases and reaches a plateau at about EO 30. Further increase in EO content seems to cause a depletion of the surfactant from the interface, which increases IFT.
The lowest interfacial tension reduction was observed with methyl methacrylate. The following order is observed in the efficiency of the surfactants:
EO < 20
methyl methacrylate>vinyl acetate>styrene.
EO > 20
methyl methacrylate>vinyl acetate>styrene

Effect of the Hydrophobic Group on Stabilizing Effectiveness at Water/Monomer Interfaces
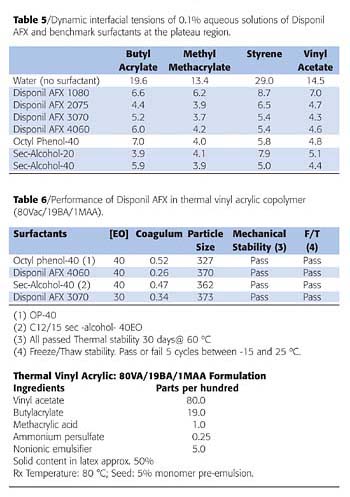
Table 5 compares the effectiveness of Disponil AFX and benchmark surfactants in reducing interfacial tensions at water/monomer interfaces, i.e., the ability of the surfactants to stabilize emulsions of methyl methacrylate, styrene and vinyl acetate. As can be seen, at a bulk surfactant concentration of 0.1%, the effectiveness of AFX surfactants is similar to that of either octyl phenol or oxoalcohol surfactants.
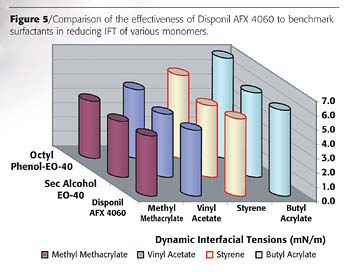
Figure 5 compares the effectiveness of Disponil AFX 4060, octylphenol-40 and sec-alcohol-40. All the surfactants compared in this study have the same ethoxylation level of 40-EO. The effectiveness is related to the maximum reduction in interfacial tension given by a surfactant. In this particular case it is related to the ability of the various surfactants to stabilize emulsions where the organic phase is either methyl methacrylate, (MMA), butyl acrylate (BA) or styrene (S). Figure 5 shows that AFX 4060 is as effective as the aforementioned monomers in lowering the interfacial tension of emulsions made with these monomers.
Performance in Emulsion Polymerization
The mechanism of emulsion polymerization as well as the role of surfactants has been discussed in a number of publications.4-12Table 6 shows the performance of different nonionic surfactants as primary emulsifiers in a thermal vinyl acrylic copolymer of composition: 80% vinyl acetate, 19% butyl acrylate and 1% methacrylic acid. The data presents a comparison between the new generation of green surfactants - Disponil AFXs - and the traditionally used APEs and sec-alcohol ethoxylates at [Surfactant] = 5% based on monomer concentration.
The table compares AFX nonionic surfactants to a well-known APE such as octyl phenol 40-EO (OP-40). The data also compares a synthetic sec-alcohol bearing the same ethoylation level (40-EO).
The results show that OP-40 produces somewhat smaller-particle-size latexes than the AFXs; however particle size can be effectively controlled by varying the surfactant concentration. The AFXs gave cleaner latexes with acceptable mechanical, electrostatic and freeze/thaw stabilities. This data suggests that Disponil AFX emulsifiers are excellent candidates to replace APEs and synthetic sec-alcohol ethoxylates.
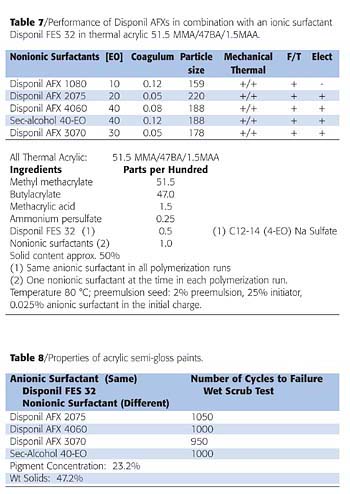
Surfactant screening in model formulations has shown that Disponil AFXs are also effective emulsifiers for acrylics. Table 7 shows the performance of combining each nonionic emulsifier with a short-chain alkyl ether sodium sulfate called Disponil FES 32. The results demonstrate the effect of surfactant combinations to control particle size, overall stabilization and low coagulum content. The AFX surfactants produced clean latexes with controllable particle sizes and acceptable overall stability.
Although the latex made with the low-molecular-weight Disponil AFX 1080 gave a desirable small particle size, the electrostatic stabilization was lost. This was obtained by post-adding a small quantity of Disponil AFX 4060. This demonstrates that AFX emulsifiers are excellent during polymerization as well as suitable post additions to increase overall stabilization.
It is important to note that the coagulum formed in the latex made with the sec-alcohol ethoxylate was significantly higher than the coagulum formed in the latexes made with the Disponil AFX surfactants bearing similar ethoxylation.
Figure 6 shows the dynamic viscosity of the latexes measured using an Advance 2000 rheometer made by TA instruments. As expected, all the latexes show a typical thixotropic behavior. It is interesting to note the excellent correlation between the viscosity and the mean particle size (determined using a Nicomp Submicron Particle Analyzer). The rheology of the semi-gloss paints made with the previously discussed latexes also showed a thixotropic rheology.
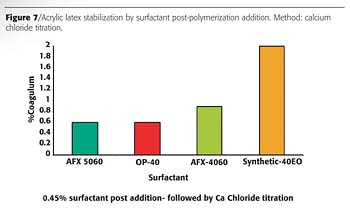
Results shown in Table 8 summarize the wet scrub cycles determined on the semi-gloss paints formulated with the acrylic latexes discussed earlier. Different paints are identified by the name of the nonionic surfactant used in the acrylic latex preparation.
It is interesting to observe the high number of cycles, which is outstanding for paints made without wet adhesion monomer/s. These high scrub cycles are indications of excellent adhesion and water resistance.
It is important to note that although the average number of cycles is not significantly different, the coagulum formed in the latex made with the sec-alcohol ethoxylate was significantly higher than in the latexes made with the Disponil AFX surfactants bearing similar ethoxylation.
The significantly cleaner latexes and high number of cycles to failure in the acrylic paints further demonstrates the suitability of AFX emulsifiers to replace APEs and synthetic alcohol ethoxylates in commercial formulations.
The traditional AP-based recommendation of surfactants in European countries was a combination of Maranil®, a 25 (linear dodecyl benzene sodium sulfonate) and Disponil NP-30 (nonyl phenol 30EO). Although the combination did not give acceptable block resistance, it provided good polymerization performance, good mechanical, electrolyte and mechanical stability. As Table 9 shows, the blocking resistance was considerably improved using Disponil AFX 3070 instead of Disponil NP-30. Further improvement was achieved using Disponil AFX 4060.
Latex Stability: Post Polymerization Stabilization
Emulsion polymer and coatings producers are increasing their efforts to replace OP-40 and similar APEs in a wide spectrum of latexes and coatings. New Canadian regulatory pressures, older regulatory European regulations and recommendations, and trends to globalize formulations are the most important drivers for the awareness and pro-actions shown by most suppliers.The purpose of this section is to discuss the role of AFX surfactants as latex stabilizers during post-polymerization processes, specifically in formulations with high inorganic filler content.
A fundamental property of the surfactant molecules is their ability to orient and adsorb at the interfaces. In latex systems, most of the surfactant is adsorbed at the interface between the particles and the water. This adsorption is a thermodynamic process favored by a decrease in the overall free energy of the system. The adsorbed layer of surfactant molecules is essential for the stabilization of latexes during polymerization and during the post-polymerization processes.
It is well known that the colloidal stabilization depends on the interactions of attractive and repulsive forces between the particles. It is also accepted that electrostatic repulsion stabilization and steric or spatial stabilization are the two most important barriers to flocculation. The addition of inorganic fillers containing divalent metallic ions, such as calcium, magnesium, zinc, etc., produce rapid flocculation by disrupting the electrical double layer surrounding the polymer particles. Thus, reducing the electrostatic repulsions between the adsorbed anionic surfactant molecules typically used during the emulsion polymerization process. A pragmatic remedy to restore stability is the addition of high-HLB surfactants, specifically polyethoxylated nonionic emulsifiers. The hydrophilic polyethoxylated chain provides steric stabilization by orienting and extending towards the water phase. This prevents the particles from entry into the effective space of mutual attraction. Also, it has been proposed that the effectiveness of this type of stabilization is due to the hydrogen bond interactions and high localized viscosity of the hydrated polyethoxylated chain, which acts as a protective shield preventing flocculation.15-18
As summarized above, high-HLB nonionic surfactants are characterized by low amounts of surfactant molecules adsorbed at the interfaces and high cross-sectional areas at the interfaces. These properties make them ideal as post-polymerization stabilizers.
High-HLB Disponil AFX surfactants have shown excellent performance as post-polymerization emulsifiers to improve the electrostatic stability of commercial latexes, including acrylic copolymers as well as styrene butadiene copolymers. Post-polymerization stabilization is important for latex formulations with high filler content such as traffic paints, architectural coatings, paper coatings, etc.
Disponil AFX 4060 and Disponil AFX 5060 were evaluated as post-polymerization stabilizers and compared to OP-40, and a C 12-15 synthetic sec-alcohol 40EO.
In this discussion, the latex stability to divalent cations and hence the electrostatic stability of the system, is measured by the stability of the latex to calcium chloride titration. The evaluation of the latex stability consisted in measuring the coagulum formed in a model acrylic latex after the addition of certain amount of surfactant, followed by the addition of 5% aqueous solution of calcium chloride. The model acrylic latex was specially designed to lack calcium chloride stability. It was made with Avirol® SL 2010 a sodium lauryl sulfate surfactant as the primary anionic emulsifier.
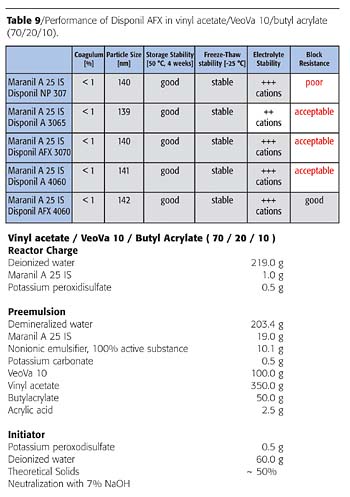
The results of the post-polymerization stabilization study are shown in Figure 7. The minimum amount of nonionic surfactant necessary to restore the electrostatic stability of the latex was determined to be 0.45 wt.%. The stabilization efficiency of each post-added emulsifier was evaluated by measuring the coagulum formed upon titration with 5% aqueous calcium chloride solution. Disponil AFX 5060, OP-40 and Disponil AFX 4060 produced the lowest amount of coagulum. The amount of coagulum formed in the latex containing the synthetic sec-alcohol was more than twice the amount of coagulum formed by post adding the Disponil AFXs. These results indicate that AFX 5060 and AFX 4060 are good alternatives to replace alkyl phenol ethoxylates.
It is important to note that higher concentrations of post-added emulsifiers may give similar low coagulum formation. However, in order to establish their efficiencies and differentiate between various emulsifiers, it is critical to determine the minimum amount of surfactant required to restore stability. For example, at 1% surfactant post-addition the coagulum formation was very small, and no differences in stabilization performance could be determined. Below 1% the stabilization efficiencies were significantly different, as shown in Figure 7.
It has been observed that the efficiency and effectiveness of surfactants as post-polymerization stabilizers is system dependent. Certain latexes made with large quantities of anionic surfactants as the primary emulsifier may require more than 1% nonionic surfactant addition to restore the electrostatic stability.
Summary
The experimental results presented in this paper illustrate the efficiency and effectiveness of the new generation of nonionic surfactants Disponil AFX. With varying degrees of ethoxylation, they can be used in a variety of applications, either to effectively improve adsorption at diverse surfaces and interfaces or to efficiently stabilize emulsion polymers. In sum, this new generation of green nonionic surfactants is mostly based on renewable resources; they are readily biodegradable and exhibit low toxicity. These characteristics make them environmentally acceptable and provide an opportunity for developing new formulations that are free of harmful European labeling. They are clear high-solids liquids, easy to handle at ambient temperature and can be used to make high-performance commercial latexes and coatings. The Disponil AFX surfactants enjoy the same FDA approvals as the Disponil As. They are approved for use in paper and paperboard, and adhesives under 176.170, 176.180, and 175.105 respectively. Disponil AFX emulsifiers have shown to be effective during polymerization and as post-polymerization stabilizers, replacing high EO bearing APEs and synthetic sec-alcohol ethoxylates.
Acknowledgements
The authors are grateful to Sandra Heldt, Thomas Mausberg, Ronald Lafreeda and David Digiulio for their help in the laboratory formulations and tests.
References
1 Swisher, R.D. Surfactant Biodegradation. Marcel Dekker, 1987.
2 Kravetz, L., et. al. JAOCS, 68, 1991.
3 White R., et.al. Endocrinology, 135, 1994.
4 Harkins, W.D. J. Am. Chem Soc. 69,1428, 1947.
5 Smith, W.V.; Edwards, R.H. J. Chem. Phys. 16,592,1948.
6 Smith, W.V. J. Am. Chem. Soc. 70,3695, 1948, ibid. 71,4077, 1949.
7 Blackley, D.C. Emulsion Polymerization. Applied Sci. Publishers Ltd., 1975.
8 Fernandez, A.M; Evans, E; Held, U;Feustel, D; Natale, M; Tuttle, B; Klima, R. International Waterborne Symposium, 1999.
9. Piirma, I. et.al. J. Polym. Chem., 20, 489, 1982.
10 Piirma, I. et.al.polym. Bulletin, 11, 497, 1984.
11 Medvedev, S.S. et.al. J. Macromol.Sci. Chem., 7,715,1973.
12 Okamura, S., et. al. J. Polyms. Sci., 58,221,1962.
13 Rosen, M. Surfactants and Interfacial Phenomena, 2nd Ed, John Wiley & Sons, 1988.
14 Saint Victor, M.E. Proceedings 94th AOCS Conferences, 2003.
15 Verwey, E.J.W; Overbeek, J.G. Elsevier, 1948.
16 Naper, D.H. Sci. Prog. Oxford, 55 , 1967.
17 Napper, D.H. Trans. Faraday Soc., 64, 1969.
18 Napper, D.H. J. Colloid. Interface Sci. 32, 1970.
Presented at the Federation of Societies for Coatings Technology Annual Meeting Program, October 2004.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!




