Upcycling from Water Bottles to Protective Coatings
A New Approach and a New Life – Part II



Author’s note: Part 1 of this article appeared in the November 2015 issue of PCI.
Depending on the substrate being protected and the environment it is being protected from, recycled poly(ethylene terephthalate) (rPET) can be an excellent starting point for designing high-performance protective coatings with long service lifetimes. Starting from a discarded water bottle that was once on a supermarket shelf, the transition to such a protective coating would qualify as “upcycling,” as opposed to a classic recycle category, or the more commonly practiced “downcycling.” Most plastic recycling is classified as downcycling by McDonough and Braungart,1 meaning that the process reduces the quality of a material over time. The practice of upcycling then would logically refer to improving the quality of a material over time. This can be accomplished with rPET by nominal digestion followed by a chemical rebuilding process, which brings about an entirely new material with desired properties and performance. It contains the contribution from a significant fraction of rPET incorporated within the new structure, but has been re-engineered for a new functional role. It has been upcycled. Upcycling and downcycling are both sustainable processes, however, the former provides greater value for the end product than the latter.
This article describes some recent work carried out in our laboratories with coatings that are based on upcycling of both PET and poly(bisphenol A carbonate) (PBAC). These two recycled materials have been combined to create interesting hybrid compositions for direct-to-metal (DTM) applications. Along with high-performance characteristics, these new compositions for coatings are highly sustainable based on their “green” content. They also represent another example of how the coatings industry is embracing the challenge of environmental responsibility coupled with long service lifetimes for both the coating itself and the substrate it is designed to protect. By protecting durable goods from environmental damage, such as corrosion, this ultimately prolongs the lifetime of the goods. Thus, sustainability in coatings promotes overall greater sustainability in general.
With the current awareness around sustainability, especially for chemical processes, we use the term here to represent three dimensions. These are the reduced effects on human life, on the environment and on the overall energy footprint.2 We also promote the notion that all three dimensions are necessary for a true “sustainability” claim, with the term “green” acting as a method or material. Sustainability is a process and a goal, and using green input helps us reach that goal. To that end, we have made new coating resins by tapping into a vast material resource that is extracted from a waste stream headed to the landfill, the ocean or the incinerator. All of the usual chemical processes used to derive the original ingredients for making the material have therefore been circumvented by directly using recycle streams. And finally, the energy footprint required to produce coating products from these recycled streams is substantially less than that required to make the same products starting from virgin ingredients. With this approach, along with some unique combinations of materials and new processes, we’ve taken recycled usage to a new performance level, while bringing a huge sustainability claim; a claim that also includes some newer green processed ingredients.
In keeping with our company’s overall mission, the chemical rebuilding of polyols from recycled materials is based on a tiered ingredient selection process. The best coating performance from a polyol is targeted by first selecting from recycled materials, and, if necessary, then selecting ingredients from a biorenewable source to maintain the highest green composition possible. The overall green measurement of a product is defined here as the combination of contributions from the recycled and the biorenewable components. When necessary, petroleum-derived ingredients are moderately incorporated, when other choices will not enable the level of cost/performance needed for a specific function. In this way, the green content is maximized, while simultaneously meeting or exceeding the desired performance criteria, including a market-neutral cost position. In other words, it should not, and does not, validate a premium charge to customers wanting performance along with making an environmentally conscious choice.
Selection of Materials
Polyol materials for this work were selected as described earlier3 from a wide array of performance attributes. The performance characteristics were compiled using a simple algorithm to rank 67 experimental polyols based on total scores from 1K baked film test data (hardness, flexibility, adhesion, etc.). From the combined scores, the top one-third performing polyols were next screened for their performance under salt spray conditions, and several winning compositions were obvious from the group. As it turned out, the chosen selections were all based on hybrid polyols from rPET and recycled PBAC (rPBAC).
A previous paper3 on this topic described the results from two separate external laboratory evaluations of these materials. The first was a 1K study using melamine at 15% and 25% in 1 mil white films baked at 232 °C peak metal temperature. Performance was based on physical testing of the cured films, however, they were unable to test any environmental exposure conditions. In the second study, similar materials were formulated into 2K coatings that underwent a short bake at a much lower temperature (130 °C). These films were applied at 2 mil dry film thickness (DFT), tested for coatings performance, and evaluated by both salt spray and QUV exposure. Corrosion performance was excellent as compared with a commercial control paint.
Here, in the third segment, we revisit the 1K coil application, using a third external laboratory with strong expertise in the coil application area. We again looked at melamine-baked films, this time with 18% melamine at a significantly lower dry film thickness. The films were evaluated for coating performance as well as several environmental exposure tests. The new data were very consistent with the trends from previous studies.
Experimental: Coil-Bake 1K Low-Gloss White Metal Topcoat
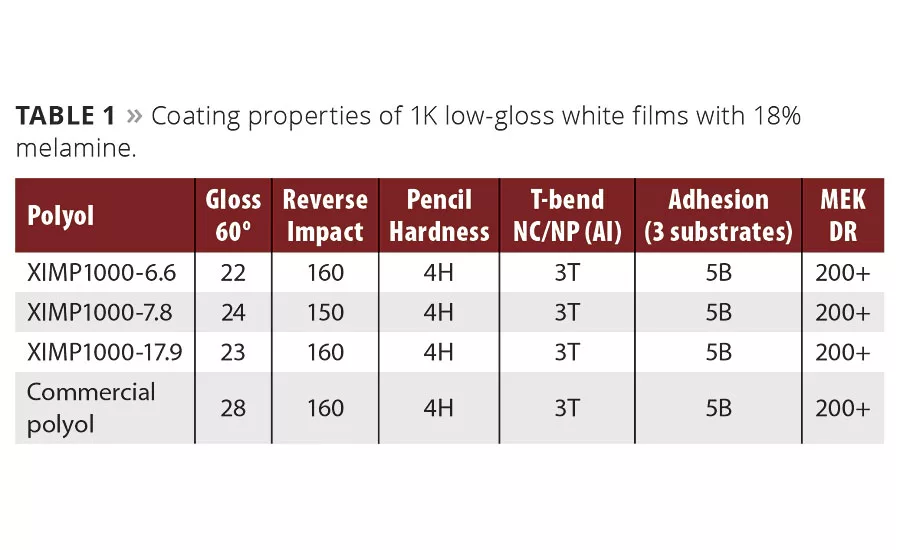
As a final validation of the performance advantages seen so far, we contracted with Chemical Dynamics, LLC in Plymouth, MI, for a more detailed look at the 1K melamine system in a coil coating application. There were three polyols evaluated as low gloss, white, one-coat formulations over chrome-treated aluminum, hot-dipped galvanized (HDG), and zinc-phosphated cold-rolled steel (CRS). The crosslinker was hexamethoxymethyl melamine, used at 18% on solids, catalyzed with 0.2% blocked DNNSA catalyst. The sole pigment was TiO2 at 22% pigment volume concentration and a pigment/binder ratio of 1.0. Formulations used Gasil HP270 silica gel matting agent to a 30 gloss reading at 60°. The bake targeted a peak metal temperature of 435-450 °F with a high airflow Blue M oven set at 550 °F, and the final film had a DFT of 0.65-0.70 mils. In conjunction with the experimental paints, a standard commercial polyester polyol was obtained, formulated in the same manner and subsequently tested after an identical bake schedule. The commercial polyol chosen was a well-known polyester polyol currently sold and used in the coil coating market.
Results and Discussion
For this third study, paints were successfully formulated for the coil application using three experimental polyols along with a commercial benchmark polyol. The paints had similar viscosity and solids, similar application properties, and three of the four had similar color. The film properties were equivalent for pencil hardness, adhesion, reverse impact, T-bend flexibility, MEK resistance, QCT humidity, and salt spray over pre-treated aluminum substrates. The Resinate polyol was found to outperform the commercial control for carbon oil stain resistance, salt spray over HDG and phosphate-treated steel. The control polyol offered better gloss and color retention upon exposure to QUV-A 340. The baked film properties are shown in Table 1. There was very little differentiation in performance at this point.
On the chrome-treated aluminum panels at 1000 hrs salt spray exposure there was no evidence of corrosion, all panels rated a 10 for field blistering4 and field corrosion,5 as well as a 10 rating for the scribe.6 Therefore, all visuals were a 1 rating. This data validated performance of the new polyols against a known benchmark. The fact that there was no differentiation supports usage in applications over aluminum substrates.
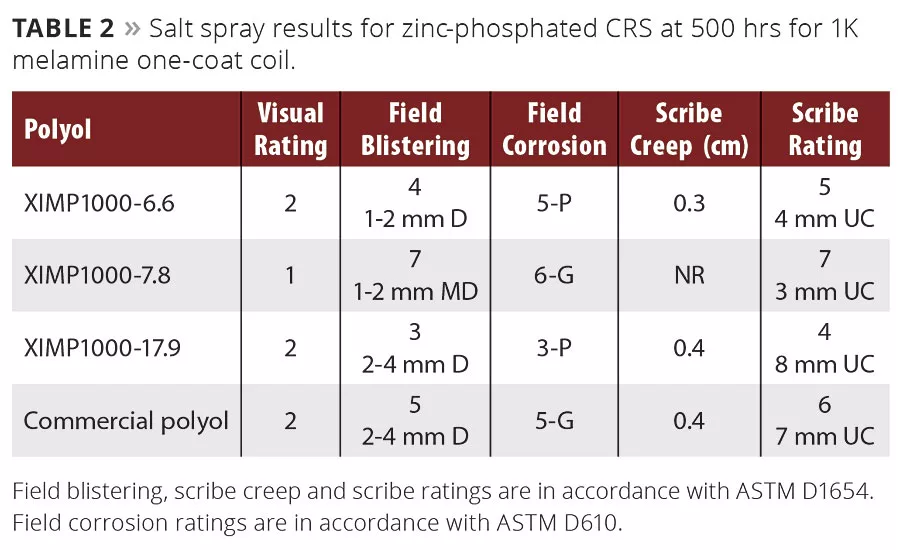
The salt spray performance data for the zinc phosphate-treated CRS at 500 hrs is presented in Table 2. The visual rating of 1 was given to a Resinate experimental sample, having the best performance at the scribe and in the field. We had observed this in the prior study using the 2K paints, and this was consistent with that performance. However, in each case we outperformed a different industrial benchmark. In this study, the paint formulation used was specific for incorporation of the commercial control polyol, and the experimental polyols were simply substituted into the same formulation. The paint with the commercial polyol was intended specifically for coil application, and as the results indicate, one of the experimental polyols clearly outperformed it in this screening.
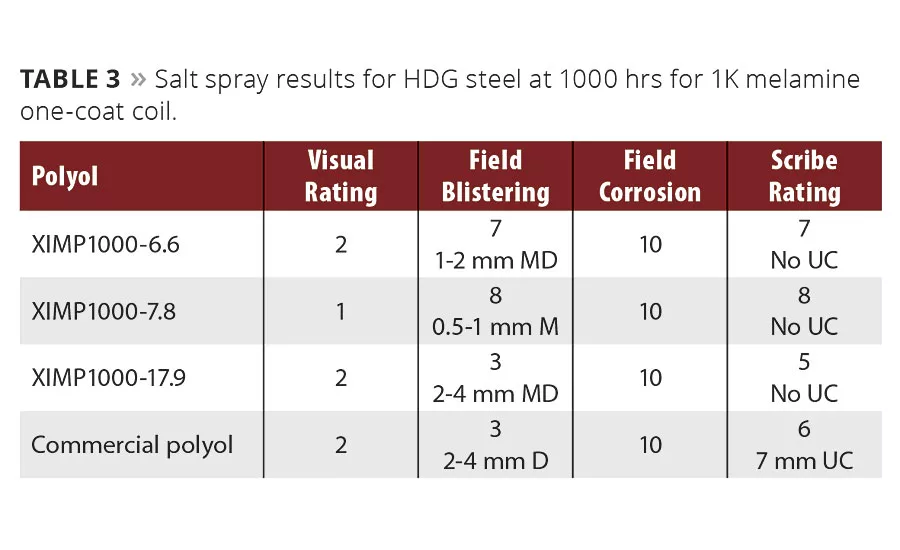
We saw similar relative performance at 1000 hrs over the HDG substrate, shown in Table 3. Although all the paints performed well over this substrate, there was enough differentiation to assign a 1 visual rating to the best experimental polyol from Resinate once again for having the lowest level of field blistering and the highest scribe rating, along with no undercut seen at the scribe. This trend has held consistent throughout the separate independent studies.
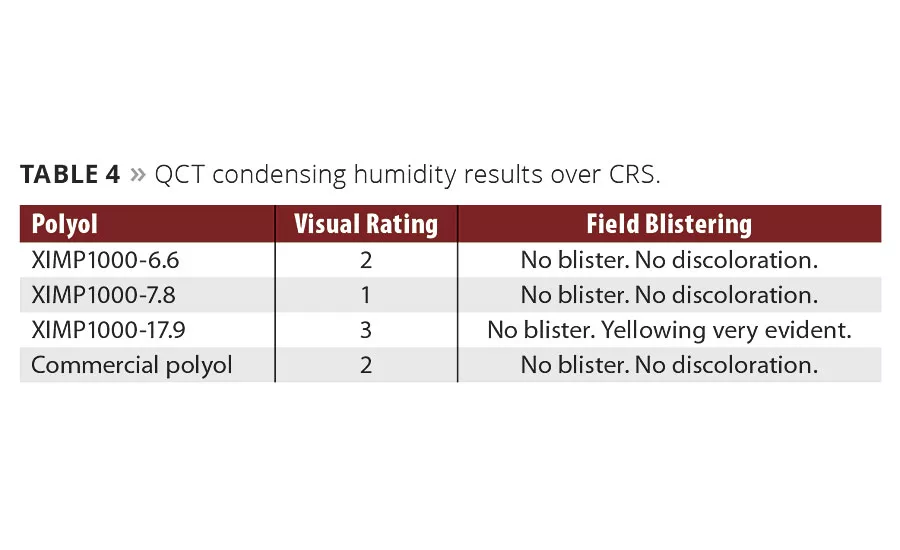
In addition to the above data, Chemical Dynamics also looked at the QCT 120 °F condensing humidity test, (ASTM D4585), to verify that our polyester-based films would not hydrolyze and/or blister after 1000 hrs of exposure. The results from this exposure are shown in Table 4, where the same Resinate experimental polyol again outperformed the same control paint with a visual rating of 1. There was one experimental polyol that displayed some yellowing (XIMP1000-17.9), but the other panels did not appear to be affected as indicated by either blisters or discoloration.
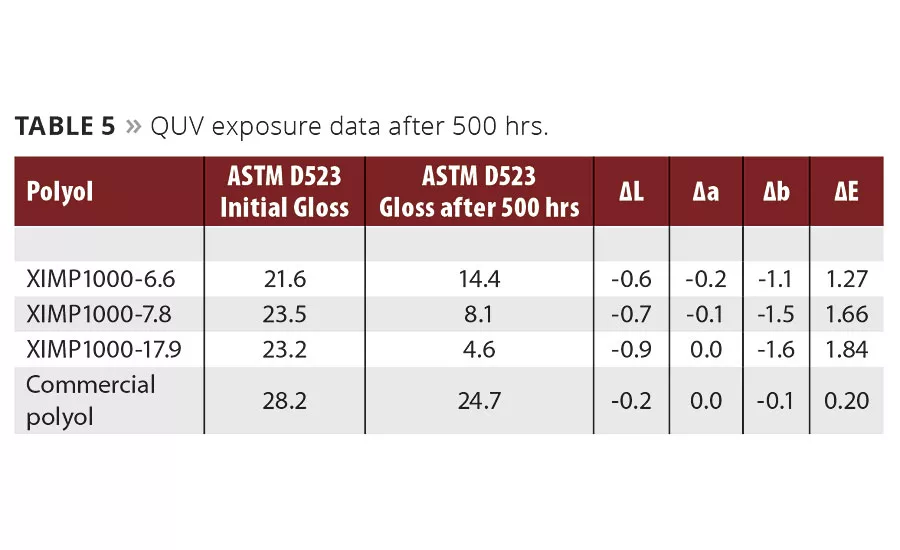
To assess the vulnerability of the aromatic experimental polyols toward exposure to outdoor elements, Chemical Dynamics assessed QUV-A340 exposure using ASTM D4587 Cycle B testing protocol. This used both a wet and dry cycle along with the UV exposure. The change in gloss was measured after 500 hrs on steel panels, as well as the color values and changes. The resulting data is shown in Table 5. It is clear that the experimental materials based on high aromatic content have greater susceptibility to UV damage and gloss loss, based on the results. The experimental polyols did not have any additive protection through hindered amine light stabilizers (HALS) or UV absorbers since they degrade at high bake temperatures. A topcoat over the experimental films would protect them much more as a primer, and might be a better choice for outdoor use. A full study on potential stabilizer packages to implement in the paint formulation for use in exterior topcoat applications has not yet been investigated.
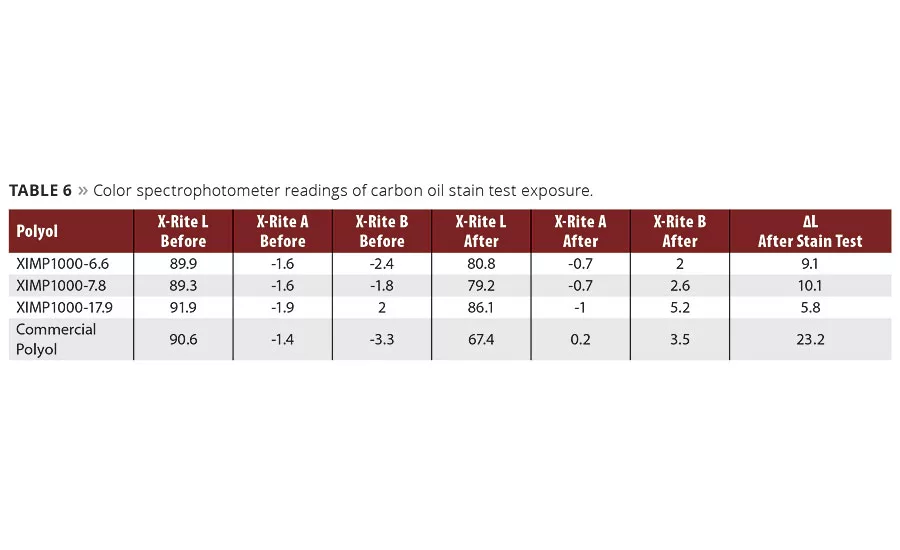
The coated panels were also exposed to a carbon-black/oil mixture spot test to evaluate their sensitivity to permanent staining, based on the change in the lightness/darkness reading from a spectrophotometer (DL) (Table 6). The Resinate material did surprisingly well in this test, again outperforming the commercial control (Figure 1). This property was not intentionally designed into the product, but was a nice bonus advantage. A reduced tendency for staining has been associated with better overall performance on sheet coated for various outdoor electrical appliances7 (air conditioners, heat pumps, water heaters) and gutters, where staining from rain was most conspicuous.
Summary
The commercial control in this study has been a widely used polyol for many years in both coil primers and coil topcoats as it offers an excellent balance of performance properties. The RMG resin platform has proven viable as a one-coat over multiple metals with the strongest performance in corrosion and stain resistance and the weakest performance in accelerated weathering. Accordingly, to capitalize on the strong attributes of the RMG rPET resin platform, two potential applications include coil primer and interior topcoats (with primer as well as one coat DTM). Coil primers are used in multiple applications including interior and exterior applications (e.g. building side walls and roofs, metal purlins, metal furniture, hot water heaters and furnace wrappers, appliances, file cabinets, T bars for drop ceilings, metal doors, automotive underhood parts and oil filters). In the proper formulation it may be possible to capitalize on the new, improved corrosion resistance by replacing a two-coat system with a one-coat product. Additionally, formulating a chrome-free primer to replace a chrome-containing primer could be successful for use on steel surfaces such as CRS, hot-rolled steel, galvalume, hot-dipped galvanized steel, electrogalvanized steel and aluminum. As some of these applications will require white colors, the formulation of a chrome-free primer using this newly demonstrated technology should take precedence.
Overall Conclusions and Future Work
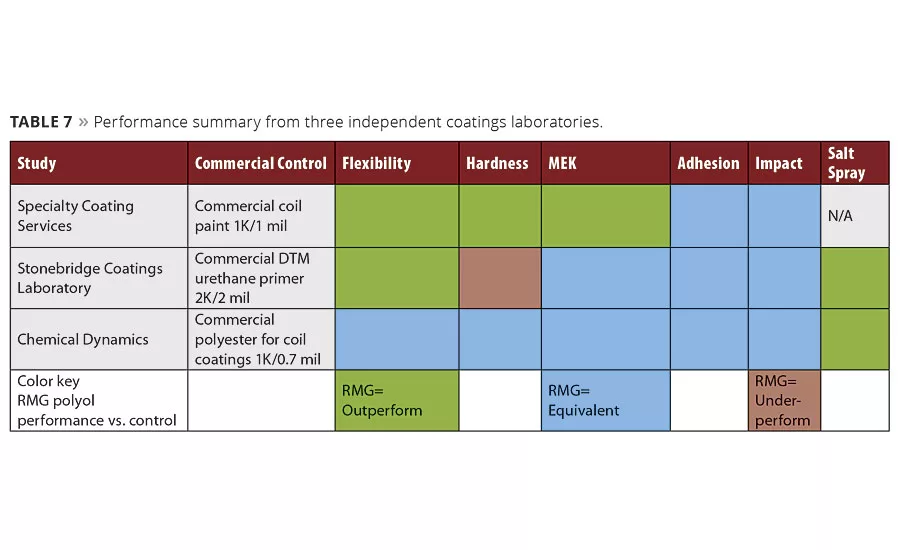
The collective work presented in this article and in the previous article is summarized in Table 7. It shows a snapshot of conclusions from three different independent coatings laboratories, which indicate consistent advantages associated with using RMG products vs. several commercial benchmarks. This work has validated a place for some novel new polyols with high recycle content combined with high performance for specialty coating applications, where protection of metal surfaces is paramount. The properties associated with the above materials tested are very appropriate for today’s protective coating demands over valuable substrates. As we upcycle materials from water bottles to protective coatings, broadening the source of water bottle materials from just PET to include others has proven both advantageous and rewarding. Introducing rPBAC to the metal coatings market has provided a new and very effective method for providing corrosion protection without the use of specialty additives.
Future work will include more in-depth studies to fully leverage the performance advantage from this technology on various metal substrates. The rPBPAC/rPET hybrid system is also being further optimized for a larger number of coating systems to expand the available coating application space. Other potential applications using the Resinate polyol platform for interior topcoats will be explored in the future. With the inherent moisture and stain resistance properties there are multiple markets where the product could fit. A detailed study on potential stabilizer packages and/or siloxane-modified versions for use in exterior applications will indicate the potential of these hybrid polyols for exterior protective metal coatings.
Additionally, we have very recently found new alternative anticorrosion technology, which has indicated a similar level of performance over CRS substrate (Figure 2). This new technology relies on the synergies from two unique additives, which do not individually provide additional protection when used with a polyol. The combined effect is quite substantial, and we expect much more information to be developed and shared in future publications on this topic.
Acknowledgements
The authors would like to acknowledge the assistance of our colleagues Rick Tabor, Shakti Mukerjee and Woo-Sung Bae.
References
1 McDonough, W.; Branugart, M. Cradle to Cradle: Remaking the Way We Make Things, 2002, North Point Press, p. 56-57.
2 Spilman, G.; Emerson, A.; Beatty, M.; Brown, M.; Christy, M.; Kovsky, J.; Rogers, K.; Vrabel, E.; Tabor, R. Coatings Tech12 (6), June 2015, pp. 40-49.
3 Spilman, G.; Tabor, R.; Emerson, A.; Brown, M.; Christy, M.; Hense, D.; Jackson, M. Upcycling from Water bottles to Protective Coatings – A New Approach with New Performance – Part I, PCI Magazine, Nov 2015, pp. 26-30.
4 ASTM D714-02
5 ASTM D610-08
6 ASTM D1654-08
7 Toshin, K.; Ueda, K. Nippon Steel & Sumitomo Metal Technical Report No. 108; March 2015.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!





