Modifying Natural Processes to Produce High-Quality Iron Oxide Pigments

For centuries mankind has utilized naturally occurring iron oxides as coloring agents. It is only in the last century that people have developed ways to chemically synthesize these natural reactions. More recently the Hoover Color Division of CATHAY INDUSTRIES has worked in conjunction with iron oxide recovery to modify a naturally occurring process to produce a series of unique iron oxides pigments. Not only is the EnvironOxide® range a good source of color, it is also a by-product of a successful reclamation project.
Most people recognize that sulfur is a major contaminant in coal. As coal is burned, sulfur is released into the atmosphere where it combines with moisture to produce acid rain. Coal with even higher sulfur levels is unsaleable and left at the mines in overburden piles. What most people fail to recognize is that the sulfur in coal is not elemental sulfur, but an iron sulfide that is more commonly referred to as the mineral Fool’s Gold. When Fool’s Gold comes into contact with water it dissolves, becoming sulfuric acid. A serious environmental condition is created when this acid migrates into streams and rivers, killing off both plants and animals.
Sustainably Sourced Iron Oxide
This hazardous acid, however, does have an unseen benefit. The iron from the Fool’s Gold is dissolved in the sulfuric acid. If the acid is properly neutralized, it will precipitate out as a hydrated iron oxide yellow, which can be utilized as a pigment. Just like other naturally occurring yellow iron oxides, EnvironOxide Yellow can be heat-treated or “burned” to produce a red color (Figure 1). Unlike mined iron oxides, which consume a specific mineral deposit or a synthetic chemical process that consumes raw material, the EnvironOxide process as observed over the last 60 years appears to be completely sustainable.

Our pilot facility is located on the campus of a small American university. In the 1950s the Chemistry Department started to analyze the acid mine seep on campus. At that time the seep was measured to contain 58 ppm iron. More recent observations measure in the iron content at 56 ppm range. Therefore, it is safe to conclude that iron oxides from these sources are sustainable for the foreseeable future.
Color Strength versus Visual Differences
Being a pure iron oxide, these products give the same lightfastness and chemical resistance that people have come to appreciate from iron oxides over the centuries. The major difference between the typical, synthetically produced iron oxides and this new range of products is that the latter are significantly smaller in primary crystal size, thus more transparent (Figure 2). This is not a unique trait among iron oxides. Umber’s and sienna’s transparency have been used for centuries in transparent wood stains. Today, high-end synthetically produced transparent iron oxides are manufactured for demanding applications, such as automotive coatings.

Conventional, synthetically produced iron oxides are produced to be opaque. One traditional measurement of the pigment’s quality is its color strength when tinted with TiO2. This is an indirect measurement of its chemical purity and its apparent surface area after a given level of dispersion energy is applied (Figure 3). Being transparent, traditional color strength measurements for transparent pigments are misleading. We can achieve opacity with transparent iron oxides by increasing the pigment loading or combining it with a high-opacity pigment like TiO2. We can also reduce the loading of a conventional opaque iron oxide to achieve the same contrast ratio as a transparent iron oxide. Unfortunately, neither approach really measures the pigment quality, especially the quality of its transparency. Following conventional wisdom, all of the transparent iron oxides in the above example are weaker than a similar colored opaque iron oxide, despite similar purity and higher surface areas. Of course, one would expect a transparent pigment to allow more TiO2 to reflect light. So, is that really strength or the visual effect of the color differences that we are seeing?
The Process
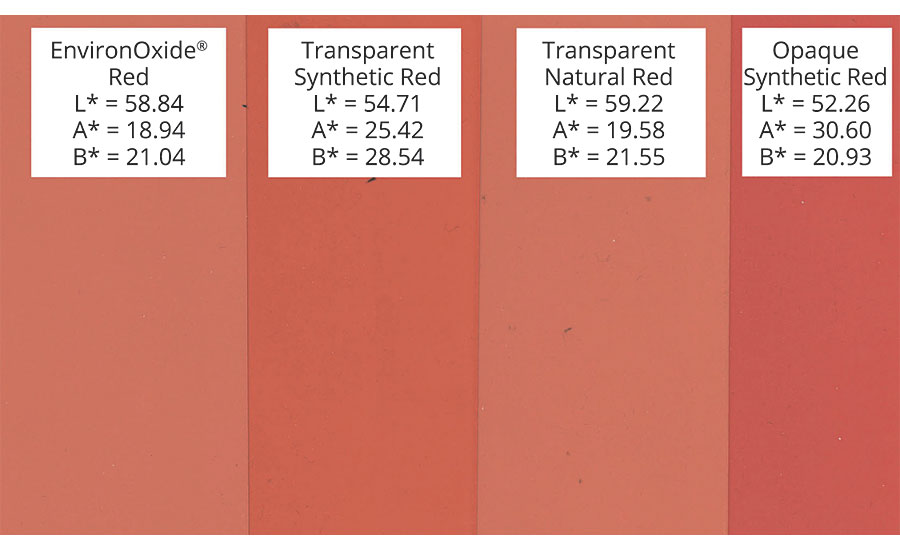
Today’s spectrophotometers utilize a contrast ratio to evaluate how opaque a coating is. It measures a coating over a white and a black substrate. The closer the color measurements are, the more opaque the coating is. So often the inverse is used to determine the transparency of a coating. But visually this is also misleading. At equal contrast ratio, pigments that have been designed for opacity have a visual “milkiness” when utilized in a transparent coating. As you can see in Table 1, an opaque iron oxide is “milky” when viewed at an equal contrast ratio with transparent iron oxides.
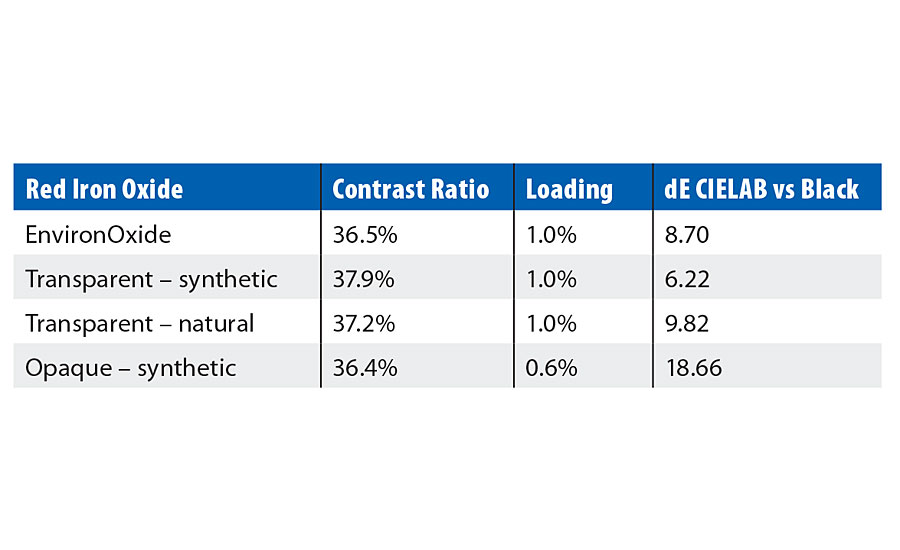
The quality of the transparency between pigments can be evaluated by using a simple dE calculation, where the standard is the black substrate and the sample is the pigment in a coating at equivalent contrast ratios. The smaller the dE* CIELAB value is, the “cleaner” the quality of the transparent color. In this example, the transparent-synthetic iron oxide has the smallest dE and visually the “cleanest” transparency (Figure 4). Of course, this is just a relative comparison, not an absolute value; but it is useful in comparing the transparency of coatings or pigments in similar coatings.

In conclusion, as an iron oxide pigment’s primary crystal decreases in size, the pigment becomes more transparent. These transparent particles can come from naturally occurring processes, they can be chemically manufactured or they can be produced from this unique process, which can be used to accomplish yellow, red, orange and brown pigments. While currently slightly “milkier” than synthetically produced transparent iron oxides, EnvironOxide products are “cleaner” than the traditional umbers, siennas and ochres in a transparent coating. In wood coatings this “milkiness” difference is unrecognizable because of the nature of the substrate. At higher loadings or in combination with TiO2, this product range can be used as part of eco-friendly opaque coatings systems. Red shades also have the heat stability needed for powder and coil coating applications (Figure 5). Therefore, these products are ideal for coating systems in pleasing earth-tone colors.

For more information, visit www.cathayindustries.com or http://hoovercolor.com/index.php/literature/sales-literature.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!



