There Are a Number of Advantages of a Starve-Fed Emulsion Polymerization Process


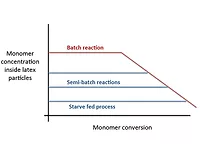
Commercial emulsion polymerizations are typically run as semi-batch processes, where the monomers and possibly other formulation components (initiator, surfactant, chain transfer agent) are slowly added to the reactor over the course of the polymerization. In contrast, in a batch process all of the monomers and other formulation components are added at the beginning of the process. The primary reason for operating in semi-batch mode is safety; the total monomer holdup at any time is low, so in the event of an accident (e.g. loss of cooling), there is no serious safety risk from a runaway reaction. However there are other important advantages related to the latex properties that can be achieved with a semi-batch process, and more specifically a starve-fed process, which is simply a semi-batch process where the monomer concentration inside the polymer particles is kept very low by keeping the monomer feed rate to the reactor low. The figure below illustrates the different profiles of the monomer concentration inside the polymer particles for batch reactions, semi-batch reactions and starve-fed conditions.
Maintaining low monomer concentration inside the polymer particles also decreases the likelihood of secondary nucleation, as there is also less monomer in the aqueous phase. Secondary nucleation alters the desired particle size distribution and can cause stability problems. With less monomer in the aqueous phase, there is also less tendency to make water-soluble polymer or oligomers, which can also cause stability problems as well as change the rheological behavior of the latex.
Very few monomer pairs react with each other at the same rate, and some copolymerizations, for example acrylates or methacrylates with vinyl acetate, exhibit significantly different reactivity of the individual monomers. In a batch copolymerization of methyl methacrylate with vinyl acetate, the polymer formed early in the reaction is comprised mostly of methyl methacrylate while that produced later in the polymerization is comprised mostly of vinyl acetate. A much more uniform polymer composition can be produced with a starve-fed process; keeping the concentration of both monomers low “forces” them to react with each other and therefore gives more uniform copolymer composition over the entire reaction.
Finally, and perhaps most importantly for some applications, a starve-fed process enables the design of specific types of particle morphology, for example particles with a higher Tg (hard) core and a lower Tg (soft) shell, which gives a lower minimum film formation temperature while preserving the hardness of the final coating.
As always, we invite your questions and comments via our website, www.epced.com.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!