An Economical Way of Improving Coatings
Montmorillonite is the main constituent of bentonite and it is a smectite clay of worldwide distribution.1,2 Workers in the field have been trying to improve the miscibility of the clay in polymers for more than 100 years.3-5 It has been observed that if montmorillonite is first ionically reacted with an organic ammonium ion, the solubility of the montmorillonite in the polymer or other oil-soluble material will be increased.3-5 Many of those polymers that display a glass transition temperature (Tg) would have their respective Tg increased. The reason for this observation has been the topic of much debate and research.
The patented material, described in US patent number 6,734,229, is one of the latest of these materials and could provide to the coatings formulator options that the previous generations of organically modified clays (OMC) could not. Our process produces OMC particles that are generally on the lower end of the colloidal scale, rending them nearly invisible to the unaided eye while raising the coating’s Tg and other mechanical properties.

Experimental Test Procedures
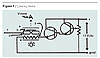
Our test polymer was polycarbonate (PC) poly[2, 2-propanebis (4-phenyl carbonate)]. Measured amounts of OMCs and PC were mixed together with the use of clean laboratory chloroform and a small amount of anhydrous isopropyl alcohol. The resulting solutions were well mixed in the divots of a clean porcelain spot plate and then set aside to allow the solvents to evaporate. The resulting OMC/PC films were cut into manageable pieces and placed in dated and labeled containers. These pieces were further prepared by heating them in a glass coverslip sandwich on a laboratory hot plate set at “medium”. These sandwiches were compressed by a wooden stick, and the process took approximately 10 seconds. The films were about the same “water clear” transparency as the parent PC.A film was then scratched or scored with an emery board or a piece of sandpaper. The scored film on a glass coverslip was placed on the heating block of our Tg testing device as shown in Figure 1. This device uses a pair of crossed polarizing films and was built by the author. This design was developed at GE by employees Dr. Allen R. Schultz and Dr. John T. Bendler. A 10-ohm, two-watt sand-type resistor [f] was used to heat the heating block [d] and hence the polymer film [b]. A centigrade thermometer, with its mercury bulb in a hole in the heating block, read the approximate temperature that was experienced by the polymer[c]. The unit was calibrated with cinnaminic acid (mp 134 – 135 oC), cholestan-5a, 6a-epoxy-3b-ol (mp 131 – 132 oC), and the Tg of unaltered PC that was found to be 150 oC.
The melting points were found to agree with published data (CRC), and the experimental Tg agreed with the PC’s product sheet from GE. The instrument was found not to need an off set. After the scored polymer was placed on a glass coverslip that was already on the heating block, the power was turned on. The light below the bottom polarizing film was adjusted for the illumination, and the power to the heating resistor was such that the thermometer began to slowly register increasing heat. The scored or scratched bit of PC through the upper film [a] was then observed. Eventually the scratch would be seen to shift or fade suddenly. The temperature registering on the thermometer was noted. Each sample of OMC/PC was tested three times in this fashion and the average temperature noted. The OMCs that we used were made with our process with docdecylamine, octadecylamine and bentonite.
Results
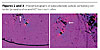
Our tests of the OMC/PC ranged from about 0.0625% to 1% w/w. See Table 1 for a summary of the experimental Tg. There was one recorded 1% averaged sample that registered as 185%, which is about 23% higher than the base Tg of PC. Evidence of the mechanism for this increase in Tgs was that the OMCs or micelles were points of nucleation for a crystallizing process (Figures 2-5). These photomicrographs were taken with a Leeds Olympus Model BX60 set to a magnification of 100 X. The instrument had a computer-interfaced camera and a rotatable microscope stage between crossed polarizing filters. The first two photomicrographs (Figures 2 and 3) are of the same PC sample with a small amount of bentonite in it (see arrows), rotated about 60 degrees from the other. The only significant birefringence is near the round air bubble. The last two photomicrographs (Figures 4 and 5) are of octadecyl ammonium OMC/PC that had been prepared in the manner given above. These last two photos show an intense change in birefringence colors suggesting that the PC has crystallized in this region of this PC film. Calculations suggested that the distances between the ammonium ions and the length of the PC monomer were similar.2,6,7Others working in this field have observed that polymers that had polar or polarizable portions in the molecular structure had also displayed changes in their respective Tgs when mixed with other versions of OMCs.4 This suggested the possibility that our OMC materials may also similarly influence other polymers besides PC.
We tested our OMCs with lacquer (without the benefit of a solvent vehicle) and found that a saturated mixture doubled the coating’s experimental tensile strength. Several brands of commercial two-part liquid epoxies were evaluated. Two samples were made in each case: one containing our OMCs and the other sample without OMC. The two samples were set aside to react or “dry”. The samples were tested with our thumbnail and a pencil. The treated samples typically seemed to have a “harder” surface and had “dried” more completely than the untreated samples. A final test was made with OMC in a toluene/anhydrous isopropyl alcohol vehicle in lacquer. Again two samples were used: the one that contained the OMC/vehicle and the sample without the OMC. A drop from each liquid was placed on a microscope slide and set aside to allow them to dry. After 24 hours the dried drops could not be visually distinguished from each other.
While our investigations were in no way exhaustive, they are consistent with previous investigations and because of this we expect that many sorts of oil-based coatings, room temperature two-part epoxies and polyesters would find our patented OMCs of use.

Conclusions
These materials can be easily made with glassware and materials that are commonly found in an organic lab and can be made in bulk, thus saving time and costs. The fact that it takes only small amounts of these materials to have such a dramatic effect on the physical properties of a coating is also an economic plus. As these OMC particles are at the lower end of the colloidal scale, current paint spraying equipment could be used with no retrofit, providing a savings for the end user.Those interested in the patent or patented materials can contact James G. Parsons, 111 Madison, #2, Rapid City, SD 57701, or via e-mail at jgp75271@juno.com. or jgp7527@hotmail.com.
References
1 Foundations of Colloid Science, Vol. I, R. J. Hunter, 1986, Oxford, pages 2 and 30.
2 Simon & Schuster’s Guide to Rocks and Minerals, A. Mottana, R. Crespi, and G. Liborio, Ed. M. Prinz G. Harolow and J. Peters, Simon & Schuster, 1998, pages 8 and 31-38
3 Structure and Dynamics of Polymer-Layered Silicate NanoComposites, Ramanan Krishnamoorti et al, Chem. Mater., Vol. 8, No. 8, 1996, Pages 1729-450.
4 Polymer Melt Intercalation in Mica-type Layered Silicates, a Ph.D. Thesis, by Dr. Richard A. Vaia, 1995, pages 4, 7, 8, 44, 18-22,24, 62, 68, and75-78.
5 Formation and Properties of Clay-Polymer Complexes, B. K. Theng, Elsevier Scientific Publishing Company, 1979, Pages 62-133.
6 Polymer Chemistry an Introduction, 3rd Ed., R. B. Seymour and C. E. Carraher Jr., Marcell Decker, Inc., pages 22, 23, 25, and 27.
7 Physical Chemistry, 2nd Ed., G. M. Barrow, McGraw-Hill Co., pages 51 and 529.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!







