Mixed Mineral Thixotropes
In the coatings industry, additive use and knowledge is always an important part of success. Little amounts can change the performance a lot. Among the widespread classes of additives the thickeners play an important role. Although they are just called “thickeners,” they are mainly thixotropes that give the coating its typical application and storage properties. The most widely used thixotropes are organoclays, fumed silicas and castor waxes. Among all applications, there are typical ones for each kind of thixotrope, defined by its inherent strength. This paper concentrates on a new kind of thixotrope, which is based originally on organoclay chemistry, but spreads out the application areas to new grounds, showing unique properties – the mixed mineral thixotropes – or, in short, MMTs.

MMTs - How They Work
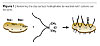
The chemical bases of organoclays and MMTs are rather similar. Both are based on clays. The anionic clay surface is covered with cationic quaternary ammonium compounds to render it hydrophobic and compatible with organic coatings (Figure 1). The chemistry of thickening is expected to be rather similar. The hydrophobic clay platelets are separated by shear during dispersion. As only the faces of the platelets are covered by hydrophobic quaternary ammonium compounds, the edges of the platelets are still hydrophilic uncovered clay.
In an organic surrounding, the hydrophilic parts of different platelets
interact with each other by hydrogen bonding. This gives a three-dimensional
network and creates the thickened gel structure, also called the card-house
effect (Figure 2). The gel has only a certain strength, which can be destroyed
by shear force and builds up again at low shear conditions (Figure 3). This is
always reversible. It gives the coating its thixotropic behaviour. Under no- or
low-shear conditions like storage and application sag, the viscosity is high
due to an intact gel. At brush or spray application the gel breaks down and
does not give much viscosity. This is why organoclays are used as anti-sag,
anti-sedimentation and anti-syneresis additives.




Application
Even though MMTs are similar to conventional organoclays and similar in performance to fumed silica, they show several advantages. First is the possibility to create higher-concentrated pregels. It is possible to produce pregels at concentrations of 10 – 15% MMT that are still pourable and easy to handle. Also not much shear is needed to disperse MMTs. In some cases circulation pumps alone can do the job. For organoclays only about 6% pregels are possible and are already on the edge of pumpability. For fumed silicas it is even much less than that. The pregels of MMTs can be made not only in solvent, but also in monomers e.g., styrene. No activator is needed, and this is an advantage in high solids (Figure 6).
The
application properties are also unique. The MMTs show a very pseudoplastic
thickening. That means low high-shear viscosity during application of the
coating, but high low-shear viscosity at rest, which gives superior anti-sag
and storage stability.

Examples
Case OneTypical systems where organoclays fail are high-solid systems. They give too much application viscosity or do not disperse at all. One system where the conventional organoclay failed is unfilled unsaturated polyester. Here the classical alternative was fumed silica. This formulation (Table 1) based on Gremopal unsaturated polyester resin is shown in a graphic (Figure 7).


The second example is also an unsaturated polyester system based on Palatal P4-01 (Table 2). In this case organoclay was not even a choice, and fumed silica could be outperformed by MMT at optimized conditions. That means, when the MMT was added as a powder, the performance was quite similar to fumed silica. Anti-sag and flow curves were rather comparable, but MMTs show a unique advantage in this case. It is possible to make a 12% pourable pregel in the styrene monomer by low shear mixing. For fumed silica there was no way to make a pregel in the existing low amount of styrene in this formulation. When the MMT was prepared as a pregel, the performance was twice as good. MMT gives the same sag resistance and a similar flow curve at 0.5 % addition level compared to 1% fumed silica. The graphic in Figure 8 demonstrates this in detail. The storage stability of the MMT formulation was the best achieved compared to all competitive products, giving no syneresis and no sedimentation.



Another challenge for organoclays are epoxy resin systems, and here especially solvent-free epoxy coatings give few possibilities to optimize the dispersibility of conventional organoclays by low concentrated pregels. For dispersing MMTs only the epoxy resin itself may work. Compared to castor waxes and amide waxes, MMTs need no elevated temperature to disperse and, of course, show no seeding problems.
An example is shown in a 2-pack epoxy floor coating (Table 3), which contains quartz sand as a filler. Due to the high density of the quartz sand it settles out quickly and the mix is not storage stable without a thixotrope. MMT was compared with a conventional organoclay and a fumed silica. The picture in Figure 10 shows the storage test results. As expected, the organoclay is not suitable at all. Fumed silica and MMT work, with advantages for the MMT.
MMTs even work in solvent-free systems; it is recommended to use a 10-15% pregel in cases were solvents are used in the formulation in order to minimize the required amount of MMT. Also monomers, or the component with the lowest viscosity, might be used to prepare a pregel. In filled systems the advantage of a pregel is often not required as there is enough milling material available to fully disperse the MMT.

Another system is shown where the conventional organoclay does not give the required performance and can be replaced by MMT. This is an unfilled 2-pack PU system. The conventional organoclays all failed in rendering the polyol part thixotropic. They just exhibit a Newtonian viscosity increase. This refers to insufficient dispersibility of the conventional organoclay. The only clay product working in the requested way is the MMT. It shows a pseudoplastic thickening. After adding the curing part, the sag was tested immediately. Also in this case, the MMT is still the product with the best sag resistance compared to all tested conventional organoclays (Figure 11).

Summary
Mixed mineral thixotropes – MMTs – show advantages towards conventional organoclays and fumed silicas. The MMTs are able to form low-viscosity, pourable pregels and show easiest dispersibility. During handling they are low dusting due to their high bulk density. The preparation of coatings is improved in terms of handling and by shorter dispersion time. Even in solvent-free high solids, MMTs are suitable as they add only little viscosity to the application viscosity, but give a large increase in low-shear viscosity. MMTs give a very shear thinning, pseudoplastic thixotropy to e.g., unsaturated polyesters and 2-pack epoxies. This leads to improved application properties like better sag resistance and better storage stability. MMTs are a powerful easier-to-use alternative to fumed silicas.MMTs are available from Southern Clay Products, Inc. under the trade name Garamite®.
This paper was presented at the Nürnberg Congress held during the European Coatings Show, Nürnberg, Germany, May, 2007 and organized by the Vincentz Network. See events@coatings.de.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!



