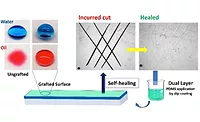
Self-Healing Polyurethane Clearcoats

Polyurethane coatings are the technology of choice for performance, beauty and low-temperature cure characteristics in automotive exterior applications. Two-component polyurethane coatings are inherently self-healing due to their unique chemistry, and they offer a number of intrinsic advantages that address customer and market requirements. These advantages include:
· high reactivity and full crosslinking, even at low-temperature curing;
· good chemical resistance and weather stability;
· hardness, toughness and elasticity due to urethane and urea structure;
· hydrogen bonds that allow for thermoplastic flow to relieve stress and allow self-healing of defects;
· basecoat compatibility;
· high solids/low VOC; and
· pleasing optical properties – a high gloss or “wet look.”
The three main drivers for product development in the coatings market today are environmental, efficiency and quality. More stringent environmental regulations encourage formulators to develop coatings with higher solids and less VOCs. To offset the rising cost of raw materials, more efficiency in the paint process is needed and has resulted in faster curing systems and the reduction of paint layers. Quality is an important requirement as customers expect a high level of performance, especially with regard to scratch and chemical resistance.
A new generation of polyurethane raw materials that produce a flexible, but highly crosslinked, polyurethane coating has been developed that meets these market drivers.
Polyurethane Coatings: A Clearcoat
An OEM polyurethane clearcoat is formed by the reaction of an isocyanate with an acrylic polyol. OEM baking conditions are 130-140 °C for approximately 30 minutes, but the reaction also takes place at lower temperatures, which means that the coating is not sensitive to underbake conditions. Polyurethane clearcoats can be used over many types of basecoats and can be formulated for high solids and low VOC.The urethane structure gives the coating good chemical resistance and weatherability, as well as hardness, toughness and elasticity. A unique aspect of polyurethane chemistry is that the hydrogen bonding acts as an additional crosslink, but also allows for the thermoplastic flow to relieve stress and enable self-healing of defects.
The basic structure of a polyurethane coating features a soft segment, based on the polyol, which furnishes the coating with flexibility and elasticity (Figure 1). There is also a hard segment that has high urethane density, which gives the coating hardness and toughness. The properties of polyurethane coatings are determined by the combination of the raw materials used in the formulation. The polyisocyanate is used in either a two-component system or a one-component (blocked) system. The polyols are hydroxy-functional materials, such as acrylics, polyesters and polyethers.

Achieving Self-Healing: The Theory
There are two main categories of scratches – marring deformation and fracture scratches (Figure 2). Examples of marring deformation include car wash scratches or any other deformation event that does not result in whitening (fracture) of the scratch trough, but still plastically deforms the clearcoat. This type can reflow when heated to a sufficient temperature. A key scratch, on the other hand, is an example of a fracture scratch. This damage cannot be reversed by exposing the clearcoat to elevated temperatures.To create a polyurethane system with the potential to recover from scratches, the crosslink density was increased while keeping the glass transition temperature, or Tg, low. (Tg is the temperature at which an amorphous solid, such as a polymer, becomes soft and starts to reflow.) Due to the flexibility of the polyurethane system, when the coating is marred the bonds are stretched - but not actually broken. When heated, such as when a car sits out in the sun, the bonds return to their original shape.


Achieving Self-Healing - In Practice
Bayer MaterialScience has developed new raw materials to make flexible, but highly crosslinked polyurethane coatings that have self-healing properties. These self-healing clearcoats have been tested by Ford using a nanoscratch tester (NST), by which a diamond stylus was pulled from left to right with increasing load. A force to fracture and a mar deformation value are measured from the scratch profile produced by the NST. For this test, an acrylic melamine silane system was utilized as a control. The results from the NST indicated that the PU system takes three-times more force to fracture than the control.Ford also used an in-house macro scratch machine equipped with four different sized tips ranging from 27 microns to 460 microns. A series of scratches were made with each scratch tip size using constant loads. The scratches shown in Figure 4 were made with the 460 micron scratch tip at a constant load of 39 Newtons and 1 mm/second scratch speed. Imagine a plow making a trough. The trough is clearly seen in the view on the left, but after reflow, the trough depth – and visibility – is significantly reduced. The reduction in trough depth is less dramatic for the control sample (Figure 5).

Application Examples
Laboratory testing was conducted on examples of automotive refinish, OEM and plastic clearcoats to demonstrate that the new polyurethane raw materials support the theory for improving scratch resistance.Automotive Refinish
An automotive refinish system was put through 10 cycles of the Amtec Kistler car wash. The gloss retention was measured after car wash testing; then the same systems were heated for two hours at 60 °C and the gloss retention was measured again.
A Bayer refinish formulation based on a polyacrylate polyol (PAC 1) and a low-viscosity trimer were used as a control. This control was compared to a formulation using a high-functionality allophanate/trimer reacted with the same polyacrylate. It was also compared with a second formulation based on the allophanate/trimer and a commercial polyacrylate (PAC 2).
When the allophanate/trimer was substituted for the low-viscosity trimer used in the control formulation, the improvement in initial scratch resistance was more than 25 percent. PAC 2, along with the new allophanate/trimer, improved the initial scratch resistance by nearly 40 percent. While there is self-healing of the control clearcoat – as demonstrated by the improvement in gloss retention of a little more than 20 percent – the recovery of the clearcoat based on the new crosslinker with the control polyol was more than 40 percent better, and with the commercial polyol more than 50 percent better than the initial gloss of the control.
OEM Clearcoat
Mar resistance and the self-healing effect were also tested using an automotive OEM clearcoat. In this case the clearcoat was scratched using a Ford crockmeter method and polishing paper. The control was a Bayer guide formulation based on an acrylic polyol (PAC 3) and conventional HDI trimer.
Combinations tested included the PAC 3 with the new allophanate trimer, an optimized polyacrylate polyol (PAC 4)/polycarbonate diol blend with the conventional HDI trimer, and with the high functionality allophanate/trimer. The initial scratch resistance improved when the conventional trimer was replaced with the allophanate trimer, and it further improved if the PAC 3 was replaced with the diol blend coreactant.
The best gloss retention was achieved with the combination of the allophanate/trimer with the PAC 4/PC diol coreactant. The recovery after two hours at 60 °C was almost 100 percent of the pre-abrasion gloss.
Plastics Clearcoat
An automotive clearcoat formulated for a plastic substrate was also put through the Amtec Kistler car wash device. The control was a Bayer guide formulation based on a polyester polyol (PES 1) with a low-viscosity trimer as the crosslinker. These results were compared with those of a formulation in which the low-viscosity trimer was replaced with the allophanate/trimer. Substituting the high-functionality allophanate/trimer for the low-viscosity trimer translated into an increase in gloss retention after initial scratching of about 20 percent, with recovery after reflow to approximately 95 percent of the original gloss.
Solvent resistance against xylene, methyl propyl acetate, ethyl acetate and acetone for these coatings increased dramatically with the substitution of the allophanate/trimer for the low-viscosity trimer. As a reference, a flexible polyester crosslinked with a conventional HDI trimer has excellent scratch resistance, but very poor solvent resistance. The new formulation provides a flexible film that has both excellent scratch resistance and excellent solvent resistance.
Conclusion
While polyurethane coatings are inherently self-healing due to unique chemistry, new raw materials developed by Bayer MaterialScience enhance this property. This has been demonstrated on examples of automotive refinish, OEM and plastics clearcoats.For example, results of Ford laboratory macro-scratch/profilometry testing demonstrate that the polyurethane system showed more scratch depth recovery versus the control OEM clearcoat. Furthermore, the nanoscratch method showed that the polyurethane system takes three-times more force to fracture than the control OEM clearcoat.
Based on these results, this is clearly a promising technology that will continue to gain momentum for various applications wherever consumers wish to retain initial quality and beauty of the products purchased.
Acknowledgements
Mark Nichols (Ford Motor Company); Patricia Jacobs and Myron Shaffer (Bayer MaterialScience); Markus Mechtel and Thomas Klimmasch (Bayer MaterialScience AG).
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!