Low-Angle Light Scattering Detection for GPC - Why Closer is Better
Successful formulation of coatings demands consideration of both the ease of application and the final surface finish. This surface finish is, in very many cases, a thin polymeric film, making polymers a key constituent of coatings products. Acrylics, polyesters, epoxy resins, polyurethanes, alkyd polymers and latexes are just some of the polymer types routinely applied to a wide variety of substrates.
The molecular weight and molecular weight distribution of these polymers are both key properties that influence ease of use and finish quality. The direct correlation of molecular weight with melting point, for example, means that for powder coatings, molecular weight must be optimized to achieve desirable flow and leveling properties during heating, without compromising the flow properties of the raw powder. Low-molecular-weight resins can soften or become sticky at relatively low temperatures, increasing the tendency to cake, which compromises spray application.
With solvent-miscible and suspension-based paints, and with varnishes, higher-molecular-weight polymers tend to be associated with a more durable protective surface, while lower molecular weights may give higher gloss and better penetration of the substrate. With solvent-miscible products based on alkyd polymers, such as gloss paints for domestic use, the incorporation of high-molecular-weight polymers is problematic because their inclusion increases product viscosity to unacceptable levels. Here, a possible solution is to introduce branched polymers: for a polymer of equivalent molecular weight, branching reduces the associated solution viscosity.
Introducing GPC
GPC (Gel Permeation Chromatography) is widely used for polymer molecular weight characterization across many industrial sectors. Traditional systems rely on a single refractive index (RI) detector and require suitable calibration standards. Such systems report relative molecular weight unless the calibration standards used are identical in nature to the polymer being analyzed, which is not always feasible.
The major attraction of light scattering detectors is their ability to measure molecular weight directly. Calibration remains necessary but is far simpler. There is no need for the specific calibration standards required with RI detection alone because the molecular weight measured is independent of polymer type or structure. Light scattering detectors therefore measure absolute, rather than relative, molecular weight. For applications where there are no suitable RI standards, such as measuring some of the extremely high-molecular-weight latexes used in water-based emulsion paints, this has an obvious and immediate benefit.
Over and above this however, combining light scattering with other detectors increases experimental productivity and gives access to more detailed molecular information. For example, the common triple detector combination of RI, viscometer and light scattering detection enables the direct investigation of polymer branching and molecular structure via the construction of a Mark-Houwink plot (log molecular weight versus log intrinsic viscosity).
The addition of UV detection to this triple system enables the detailed study of copolymers if one of the monomers has a suitable chromaphore. The technique allows the measurement of each monomer distribution as a function of molecular weight, enabling the more sensitive specification and development of polymers for a specific application.
Comparing these capabilities with the needs of the coatings industry underlines the potential value of light scattering detection for this sector.

|
| Equation 2 Click to enlarge |
Light Scattering Theory
A brief introduction to light scattering theory provides a secure foundation for assessing the relative merits of different light scattering detector designs.
Light illuminating a molecule is scattered by that molecule across a range of angles at varying intensity. Scattered light intensity and the weight average molecular weight of the molecule are linked by the Rayleigh equation, where Rθ=0 is the scattering at a zero degree angle to the incident light, Mw is the weight average molecular weight, c is the concentration of the solution, K is an optical constant that includes dn/dc, the rate of change of refractive index with concentration, and A2 is the second virial coefficient.
For dilute solutions, typical of those used for GPC, the concentration-dependent term can be ignored and the equation reduces to a simpler form. This equation directly relates scattered light to molecular weight, however, it is important to note that the equation is expressed in terms of the scattered light measured at a zero degree angle, relative to the incident beam.

|
| Figure 1 Click to enlarge |
In practice, it is impossible to measure the intensity of scattered light at a zero angle because of the intensity of the incident laser beam (Figure 1). Light scattering detectors must therefore measure at some other angle and determine scattered light intensity at zero degrees from this value. This is clearly a complicating factor.
Unfortunately the intensity of scattered light produced by a molecule varies with both the scattering angle and the size of molecule being measured. For very small molecules, scattering intensity can be considered to be independent of measurement angle, but for molecules of any significant size, (>12 nm radius)(1) the influence of angle on scattering intensity cannot be ignored.

|
| Figure 2 Click to enlarge |
Figure 2 illustrates how scattering intensity may vary, but this variation is neither constant nor readily predictable. When measuring at angles other than zero, extrapolating to zero is not always straightforward.
Choices for Light Scattering Detector Design
In the design of light scattering detectors there are three ways of overcoming the fundamental problems of having to measure an angle other than zero degrees to the incident beam. These are:
- Multi-angle light scattering (MALS). Measure the scattered light at two or more angles and extrapolate the data back to estimate the scattered light intensity at zero degrees.
- Right-angle light scattering (RALS)/viscometry. Measure the scattered light at 90º and use viscosity data to estimate the scattered light intensity at zero degrees.
- Low-angle light scattering (LALS). Measure at a very low angle, close enough to zero that the angular effects are negligible and no correction is needed.
All three options are commercially available.
Multi-angle detection avoids the technical difficulties of measuring close to the incident beam but doesn’t measure molecular weight directly. All multi-angle systems work in the same way but vary according to the number of detectors and the angles at which the light is measured. In each case measurements are taken at two or more angles and the results are then extrapolated.
It is easy to believe when using a MALS system that you are directly measuring absolute molecular weight, but this is clearly not the case. All MALS detectors require calibration and detector normalization procedures but, most importantly, the molecular weight is derived from extrapolated data, a plot of scattered light intensity against angle, extrapolated back to zero degrees.
This is the inherent weakness of the MALS approach. The choice of extrapolation fit (e.g. Debye, Zimm, Berry, etc.) and the choice of order of fit (linear, 2nd order, etc.) directly impacts the reported molecular weight, raising the question of which fit is optimal for any given sample. A broad size distribution exacerbates this problem. For small molecules a linear data fit yields the most accurate molecular weight value, but as molecules get larger a non-linear, higher order fit is more appropriate.(2) For samples containing both small and large molecules the order of fit chosen is therefore always a compromise.
The accuracy and precision of MALS detectors is further hampered because they tend to lack quality data in the all-important low-angle region. MALS detectors are designed to collect data from many angles rather than high-quality data at a single low angle. However, the goodness of fit of the extrapolation plot is, in fact, highly dependent on how close to zero the lowest angle data point is and the quality of that signal.(3) Measuring at many angles does not compensate for lower signal quality data at the smallest angles.
|
| Table 1 Click to enlarge |
RALS detectors are highly effective for small molecules when the intensity of scattered light is independent of angle. At 90º the signal-to-noise ratio is at a maximum so the quality of data measured with a RALS detector is high. However, as we have already seen, measuring larger molecules requires a correction to compensate for the variation of intensity with angle. This correction can be achieved using viscometry data where the GPC system has a viscometer as part of the detector array, but this compromises the accuracy of the Mark–Houwink plot.
On the basis of the preceding discussion it is reasonable to argue that LALS is the only one of these three light scattering techniques that lives up to the claim of measuring molecular weight directly, across the molecular size range. After initial calibration, a LALS detector measures the molecular weight of all different types of polymers directly, avoiding the assumptions and data manipulations of the alternative two methods. It is an elegant solution but one that has taken a number of years to become a commercial reality.

|
| Figure 3 Click to enlarge |
Designing a LALS Detector
The challenge of measuring scattering intensity close to the incident beam has slowed the commercialization of LALS technology. The benchmark commercial LALS detector was the Chromatix KMX-6, which was produced from the late 1970s to late 1980s. This detector was not developed further, and no new products were launched until 2001 when advances in optics and electronics allowed Viscotek to enter the market with a detector that measures at 7º to the incident beam (Figure 3).
With this clever design the incident beam is separated from the scattered light to allow collection of the scattered light intensity signal at 7º.(4) An excellent signal-to-noise ratio is achieved as demonstrated by Figure 4, which compares the LALS signal with that measured at 90º (RALS), the angle that gives the highest-quality data.

|
| Figure 4 Click to enlarge |
The data presented in Figure 4 relates to two different molecules – polyethylene oxide and hyaluronic acid. With the low-molecular-weight polyethylene oxide both the RALS and LALS detectors give the same signal because the intensity of scattered light has no angular dependence. With the larger hyaluronic acid, on the other hand, there is a very high angular dependence of the scattered light, severely compromising the RALS data. In contrast, the LALS detector is able to precisely measure molecular weight without any need for extrapolation or data fitting.
Analyzing Polymers from Coatings
The LALS detector and triple detection GPC can be employed for the full range of polymers used in coatings. Figures 5 to 7 and Table 1 show data for two of the more difficult-to-analyze molecules, nitrocellulose and polyurethane, to give an overview of the type of information produced.

|
| Figure 5 Click to enlarge |
Nitrocellulose, a film-forming resin, is used widely in many applications including paper coating and printing inks, nail varnish and automotive refinish paints. It can be a challenging material to analyze as the nitrogen content affects the polymer solubility, particularly at high molecular weight. The determination of both the molecular weight and viscosity is important for all applications, and being able to plot the relationship of both parameters (Mark Houwink plot) helps to differentiate small performance differences.
Figure 5 shows a typical triple detection GPC chromatogram with LALS of a nitrocellulose sample, and Figure 6 is a Mark Houwink plot showing the structural comparison (viscosity to molecular weight) of two samples. The structural difference revealed here can be related to the product performance.

|
| Figure 6 Click to enlarge |
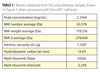
Polyurethane is used in a variety of coatings, most noticeably in varnishes, where it helps to form a hard, abrasion-resistant, durable coating. Figure 7 shows a chromatogram of a polyurethane sample together with the calculated molecular parameters in Table 1, including molecular weight, intrinsic viscosity and molecular size.

|
| Figure 7 Click to enlarge |
Conclusion
The commercial availability of LALS detectors allows GPC practitioners to measure the molecular weight of a sample with certainty, avoiding any issues of data extrapolation or data fitting. This technology is inherently superior to extrapolation of data measured across multiple angles, back to an estimated value for zero degrees. Analysis of the principles underpinning both types of detector lays bare the perceived wisdom that more angles are better. In fact it is clear that when measuring molecular weight, closer (to the incident beam) is better (LALS).
An additional important advantage of LALS detectors is that their size and simplicity facilitate inclusion in integrated multi-detector systems with, for example, viscometers, RI and/or UV detection. Multi-detector systems are a powerful option for the coatings industry, providing not only molecular weight data but also information about molecular structure and composition.(5,6) This technology maximizes the productivity of GPC experimentation towards formulation goals.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!





.webp?height=200&t=1672116434&width=200)


