The Anatomy of Multipurpose Solventborne Colourants
In the past, coatings for heavy industrial structures always emphasized protective qualities over and above decorative qualities, and were generally offered in a limited colour range. The term ‘industrial colour match’ indicated that if the supplied colour was somewhere near the same hue as the desired colour, then that was all that could be expected for a heavy-duty protective coating.
This situation rarely exists in today’s world, and the protective coating supplier is expected to approach the other, above-named markets for colour offering and quality. Concentrated pigment pastes, which exhibit compatibility with a broad range of coatings chemistry, are a useful tool in achieving this goal, and we will examine the technology of such pastes in this presentation.
Pigments
|
| Figure 1 Click to enlarge |
Pigments are naturally the heart of the pigment paste and, in addition to the obvious properties of shade, tinting strength, lightfastness etc., for colourants designed for protective coatings, one must especially be aware of solvent resistance and surface reactivity. Inorganic pigments exhibit excellent solvent resistance properties mainly due to their inherent chemistry. Their ionic crystal lattices have an interlocking arrangement of metal cations and non-metallic anions, which doesn’t allow this class of pigments to dissolve in solvents.
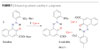
On the other hand, organic pigments exist in the form of molecular crystals where individual molecules are linked together by means of weak hydrogen bonds and π-π interactions. The solvent stability of an organic pigment depends upon the strength and extent of the interactions between the constituent molecules in its crystal lattice. In addition to this, the chemistry of the basic molecule also plays a significant role in determining the solvent stability of the resulting pigment crystals. The stability of the pigment can be enhanced by maximizing intermolecular contacts/interaction in a crystal lattice, and this can be designed into the basic structure. This is illustrated in Figure 1.
|
| Figure 2-3 Click to enlarge |
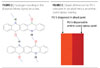
Strong bonding can also be serendipitous, as exhibited by the NH – CO interactions in the b quinacridone crystal (Figure 2).
Some pigments undergo phase change when used in combination with certain solvents. Alpha modification of copper phthalocyanine is a classic example of this. The crystal phase of this pigment is relatively unstable compared to its other polymorphs. When this pigment is used in applications containing aromatic solvents, the shade of the system will change from reddish blue to greenish blue, accompanied by a reduction in colour strength. This is due to the dissolution of the a form into the solvent and its re-crystallization into the more stable (but greener) b form. Because the re-crystallization is uncontrolled, non-ideal particle sizes can also lead to poorer tint strength and reduced durability.
Bivalent metal ions used in the lake pigments can sometimes cause gelling of paints containing carboxylic binders. This is due to the possible crosslinking of two carboxylic polymer chains through the bivalent metal ions. Improper processing of certain inorganic pigments can often create problems in systems containing reactive binders. Over grinding of such pigments results in the delamination of its surface treatment followed by the release of reactive metal ions to the continuous phase. These metal ions can sometime accelerate isocyanate/polyol reactions and thereby reduce the pot life. They can also act as driers and cause in-can skinning of alkyd paints.
Certain organic pigments can interact with reactive polymers and, for example, when used in an amine-cured epoxy system, can cause unacceptable shade changes. Pigment Orange 5 and Pigment Red 4 are examples of pigments that come in this category. The photograph in Figure 3 clearly shows the dramatic difference in shade when PO 5 was used in the colouration of an alkyd and an amine-cured epoxy paint system.

|
| Figure 4 Click to enlarge |
The treatment of pigments with their derivatives is a common practice in the pigment industry. This is carried out mainly on those pigments that have inert surfaces (i.e., have no groups or atoms that can form linkages with the surfactant or resin moieties). The idea is to derivatize the same pigment pre-cursor via sulphonation, chloromethylation, chlorosulphonation, phthalimidomethylation, etc., and attach these derivatives (or suitably converted derivatives) onto the pigment surfaces (Figure 4). This attachment is very strong, as the surface chemistry of the pigment and the molecular chemistry of the pre-cursors are very much alike.
The attachment of these groups will alter the polarity of the pigment surface, which aids in the wetting of the pigment surface by wetting agents and solvents. One can also build polymeric chains onto these derivatives to generate sufficient steric hindrance to stabilize the resulting dispersions. These pigments can be customized to one application by introducing specific polymer tails to these derivatives, or they can be made universal by linking the derivatives with more than one type of polymeric chains.
The pigment derivative of choice can be made separately and it can be utilized during the manufacture of pigment dispersions. In this case, the dispersion chemist can choose one or a combination of derivatives depending on the requirement of the dispersion in various applications. The quantity of derivatives used for the treatment is very important, as excess amount will cause solvent bleeding (owing to its molecular nature).
Pigment surface treatment is mainly carried out to optimize its performance in various applications. Certain treatments are designed to impart high performance only in specific applications, and usage of these pigments in some other area can cause unwanted results. For example, amine-treated pigments are generally easy to disperse and stabilize but can significantly reduce the pot-life of epoxy-based systems.

|
| Figure 5 Click to enlarge |
Dispersants
Pigments are highly complex materials with varying morphologies and surface chemistries. The surface chemistry may not be what one could deduce from the bulk chemistry of the pigments, as the crystallization process and/or the closely guarded ‘finishing techniques’ of the pigment manufacturer may radically alter what might be expected.
Pigments are also delivered in a highly agglomerated form that must be broken down and stabilized in order to make a useful tint paste. This is not a trivial matter, and dispersants are the most useful tool in achieving success.
Dispersants are a subset of surfactants that show usefulness in dispersing a discrete, solid phase into a continuous (liquid) phase. A very simple example is oleic acid, which contains a carboxylic ‘‘head’ that has some pigment affinity and a C17 ‘tail’, which would be expected to show compatibility with hydrocarbon solvents and oil-modified vehicles. The single carboxyl group would not be expected to show strong affinity to a pigment surface (unless the surface was very basic), and desorption leads to dispersion instability. A successful ploy is to string many carboxyls together into a polymer where it is expected that, whilst the adsorption of each single carboxyl remains relatively weak, the combined adsorption of the polyacid is relatively strong and total desorption much less likely.
Useful pigment affinic groups include carboxyls, hydroxyls (and usefully hydroxy acids), phosphates, phosphonates, sulphonates, phenyls, PEO, PPO, and a range of nitrogen-bearing chemistries including amines, amides, amino-alcohol, urea derivatives, betaines, taurides etc. One can recognize from this partial list that some of these will be reactive to commonly used protective coatings systems.
The wide variety of listed pigment-affinic groups is also a reflection of the variety of anchor sites on pigment surfaces. The greater the knowledge that can be gleaned about the nature of the surface of any one pigment, the greater the chances of making a successful pigment dispersion.
It is worth raising the question of the ability or the desirability of the coating resin to adsorb onto and thus easily disperse and stabilize a pigment. When the pigment affinic group used is not needed for film formation (such as the hydroxyl on an air drying alkyd) then the effect is desirable. But what happens if the hydroxyl is needed for reaction with an MF resin or an isocyanate? What happens to the amine group of the polyaminoamide that is adsorbed onto an acidic oxide? Are these groups available for reaction with their respective co-reactants or is the stoichiometry of the system compromised? And if they are available for reaction, does this mean that the pigment surface is stripped bare in the latter stages of film formation leading to increased porosity? Suitable stabilization with an appropriate dispersant seems to be the prudent path.
Turning our attention to the ‘tail’ of the dispersant, it is clear that it needs to be solvated by a wide range of solvents and/or be compatible with a wide range of binders. It is our observation that solvation is more important for low-molecular-weight dispersants and binder compatibility with high-molecular-weight variants.
It is also clear that no one simple A-B configured dispersant will satisfy the needs of all pigments – or even a single pigment. Let us consider the crystal shape of Pigment Red 122 (Figure 5). This crystal has 26 different facets and, because the plane of the base molecules runs on a slightly different plane to the main crystal face, each of the 26 facets can have subtly different chemistries and charge.
One can see that even if the vast majority of these surfaces could be separated and stabilized with suitable surfactants, agglomerated networks could still exist if the corners (which constitute c.1% of the entire surface) are not satisfied.
Whilst the most elegant solution may comprise a unique mixture of strongly adhering monomeric surfactants with pigment affinic groups calculated to cover the various facets of the pigment, attached to tails of varying polarity, the most likely commercial ‘universal’ dispersant will be a ‘comb polymer’ combining a variety of pigment affinic groups on one side of the ‘comb’ and a series of ‘tails’ on the other side, with repeats of differing polarities.
Dispersing Resins
If the pigment pastes are intended solely for factory use and, treated like millbases, can be stabilized with judicious amounts of binder, there is little need for the use of dispersing resins. However, when pigment pastes are post-added to various coatings, systems that are stabilized only with dispersants may not be robust enough to withstand the ‘shock’ of their introduction into the new environment.

|
| Figure 6 Click to enlarge |
The use of dispersing resins significantly increases this ‘robustness’. One cannot, however, simply dismiss the utility of dispersing resins; they also show a wide range of compatibilities with a variety of binders and also the ability to adsorb onto some pigments.
Experience shows that some classes of dispersing resin interact better with some pigments than do others, and also exhibit synergies with specific dispersants. Some regimes recommend examining systems by blending dispersant, dispersing resin and binder, and examining the cast, dried films for clarity as a guide to system ‘compatibility’. We believe this to be of limited value, as absorbed species (onto pigment surfaces) behave very differently to the unabsorbed material.
Typical dispersing resins are:
1. Ketone/Aldehyde Resins
The most well known and utilized resin of this class is the BASF product Laropal A81. This material is the further condensed product of the aldehyde formed by condensing 1 mol of urea with 3 mols of isobutyraldehyde (Figure 6).(1)
This polydisperse hexahydropyrimidine exhibits excellent compatibility with a wide range of coating resins. This compatibility may be influenced by the wide molecular mass distribution, from 500 to 35,000 daltons. It is soluble in a wide range of solvents but with only limited solubility in hydrocarbons with high levels of aliphatics. The product has low solution viscosity, pale colour, good heat resistance and good durability.
Resins based on methyl cyclohexanone show improved solubility in aliphatic hydrocarbons but generally with poorer pigment wetting, whereas resins based on acetophenone show improved pigment wetting with lower solubility and compatibility.
2. Acrylic Resins
The wide array of acrylic monomers, including functional monomers, can be used to produce resins with suitable solubilities and varying degrees of pigment affinity. Newer, sophisticated means of assembling acrylic monomers into precise formations, blocks and stars blur the distinction between acrylic dispersing resins and polymeric acrylic dispersants. Acrylics generally enjoy good durability.
3. Saturated Polyesters
The ease of condensation chemistry and the wide range of monomers available make polyesters a suitable means of assembling dispersing resins. Polyesters also provide reasonable durability but do remain susceptible to saponification.
Solvents
Solvents play an important role in the manufacture of pigment pastes as well as in their subsequent use. Solvents with mid-range solubility parameters have greatest utility, as are solvents with mid-range evaporation rates. Too high a volatility can lead to rapid drying out of the pigment pastes, whereas too low a volatility can lead to solvent entrapment in the cured film.
Blends of PM Acetate and Aromatic 100 (C-9 aromatic hydrocarbon blend) are generally favoured, but increasing pressure on the use of aromatics may result in a change away from this.
In the preparation of the pastes, the solvent is often the first thing that the pigment ‘sees’. A solvent that absorbs strongly onto the pigment surface may compete with the dispersant for that surface: a solvent that is an excellent solvent for the dispersant may hinder the partition of the dispersant from solution to the interface. The solvent blend therefore is at an optimum when it a) does not absorb strongly onto the pigment surface; b) is not a good solvent for the dispersant; and c) is a good solvent for the ‘tails’ of the dispersant.
Viscosity and Rheology
These properties, at first sight, would seem relatively obvious. At the highest pigment loading, one requires a low viscosity in the mid to high shear range to allow for easy machine dispensing. High pigment loadings often tend to dilatency, which must be avoided at all costs. Similarly, one needs high viscosity at very low shear rates to prevent pigment settling, especially for the heavy metal oxide pigments.
The main dilemma springs from the fact that liquids of similar rheological profile mix more easily with each other than liquids of dissimilar rheological profile. Shaking is often the most convenient and preferred method of incorporation, and significant mismatches of viscosity can preclude successful mixing. Compromise viscosities are sought to optimize mixing but, in some cases, the shear provided by shaking is insufficient, and mechanical stirring will be required.
Compatibility
Compatibility means different things to different disciplines. We use the following when we consider tinted pigmented coatings: a system is deemed to be compatible if the liquid components show no phase separation on a molecular scale and the solid phase fillers and prime pigments exhibit interfacial adhesion to the liquid phase, such that the whole is homogeneous on the macroscale.
This definition is idealistic and debatable. The obvious question is how are the dispersants defined? In our view, strongly adsorbed dispersants should be viewed as part of the pigment as would any other supplied ‘pigment finishing system’.
There are some obvious requirements for achieving compatibility such as the need for the solvents of the tinter paste to be miscible with the solvents of the paint system; that the solvents of the paint system are not so good that they can strip the dispersant from the pigment, nor so bad that they collapse the dispersants ‘tails’ and that the dispersing resin in the paste is compatible with the resin in the paint system at the normal levels of addition.
Mixed resin systems do not always achieve compatibility on the molecular scale but can co-exist in phase-separated domains. A simple example is an epoxy/polyamide mix, which will not achieve full compatibility until some reaction has taken place to produce a pre-curser hybrid species that will link the separate phases together. This is what happens during the ‘induction’ stage.
Where separate phases exist, the tinter paste will often show a preference for one of them, which can lead to reduced tinter acceptance. The dispersing resin can be of significant assistance in compatibilizing such systems, but often other compatibilizers may need to be added directly to the paint system. There is an increasingly wide range of block polymers that are very useful in this area, but their selection can only be sensibly done with a full knowledge of the various resins in the paint system and knowledge of the structure of the block polymer.
Effect on Final Films
An assessment of the effect of tint pastes is really an assessment of the effect of excess dispersant and the dispersing resin. This also means that pastes based on metal oxides with high pigment loading and low surfactant resin levels will have less effect than pastes based on ‘difficult’ organic pigments with the reverse properties.
For thermoplastic paint systems such as chlorinated rubber, solution acrylics and vinyls, there are very few negative side effects at typical use levels (0-10% by vol).
For thermoset systems, these excipient materials from the pastes can reduce chemical and solvent resistance in critical areas. Low level additions are scarcely an issue but, at higher levels, specific property testing is recommended.
Conclusion
The design of a successful multipurpose solventborne colourant demands a deep knowledge of the wide array of possible raw materials that can be utilized to make them, as well as knowledge of the likely systems they are to be used in. Because of the wide range of coating formulating protocols and styles used around the world, it is highly unlikely that such tinters will show the full universal compatibility that is desired. This is why the coatings formulating chemist will always be an integral part in the successful utilization of such products. Only they have the intimate knowledge of the coating formulation and the ability to ‘tweak’ it to achieve optimum colourant performance.
For more information, e-mail colin.gooch@resene.co.nz or ajith_aravind@yahoo.com, or visit www.totalcolor.co.nz.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!





