EPA's Photochemical Reactivity-Overview and Update
Photochemical reactivity is an important consideration when the Environmental Protection Agency (EPA) thinks about controlling organic solvent emissions to prevent ozone or smog formation. Because the paint industry is a heavy user of solvents, it might be valuable for paint industry personnel to become familiar with this issue.
This article summarizes EPA's current policy regarding VOC control and provides a glimpse of some of the changes that may be on the horizon. Special attention is given to the criteria EPA uses to select VOCs for exemption from control because of low photochemical reactivity.
Photochemical reactivity is a measure of how much a compound reacts in the atmosphere and contributes to the formation of ozone. Often the term is shortened to just "reactivity." It is a measure of the unique characteristics of a compound relative to its ability to form ozone. Reactivity is also affected by the characteristics of the atmosphere in which it reacts, so it is not just a function of the chemical itself. Other chemicals that may be present in the air, and the intensity of the sunlight, can affect the reactivity of a chemical. Reactivity is often used rather loosely to refer to the rate of ozone formation, the amount of ozone formed, or both.
The EPA often applies the concept of reactivity when it judges whether to regulate a VOC. EPA announced a recommended policy on control of volatile organic compounds in the Federal Register on July 8, 19771 (Figure 1). That policy recommended that state air-quality plans require control of most organic compounds as contributing to the formation of ozone.

Negligibly reactive compounds
However, the policy recognized that there are a few organic compounds that are of such low photochemical reactivity that emissions of these are unlikely to contribute to ozone formation. EPA designates these compounds as "negligibly" reactive and does not require that their emissions be controlled. In the 1977 policy, EPA named four negligibly reactive compounds. These are:- methane
- ethane
- 1,1,1-trichloroethane (methyl chloroform)
- trichlorotrifluoroethane (Freon 113).
- propane
- acetone
- methyl ethyl ketone
- methanol
- isopropanol
- methyl benzoate
- tertiary alkyl alcohols
- methyl acetate
- phenyl acetate
- ethyl amines
- acetylene
- N, N-dimethyl formamide
In addition to the compounds on the lists above, the 1977 policy acknowledged that several other compounds of negligible photochemical reactivity have been identified or implicated as being carcinogenic, mutagenic or teratogenic, or which cause other adverse health effects, such as forming strong eye irritants under irradiation. The policy stated that in view of these circumstances, it would be inappropriate for EPA to encourage or support increased utilization of these compounds. Therefore, they were not recommended for exclusion from control.
Ongoing review
The policy notice stated that as part of its continuing program, EPA would review new information relative to photochemical reactivity and where appropriate make additions or deletions to the list of negligibly reactive compounds. Over the subsequent years, EPA has made several additions to the list of compounds considered negligibly reactive and thus exempt from control. Currently there are over 40 compounds or classes of compounds on the negligible reactivity list.In 1992, EPA adopted a general definition of VOC, which was to be used in EPA's regulations governing the preparation of state implementation plans (SIPs) which are the states' plans to control air pollution. (This definition can be found in Title 40 of the Code of Federal Regulations at part 51.100, paragraph s.) This definition incorporated the list of negligibly reactive compounds and says that those compounds are not to be considered to be VOCs for regulatory purposes. The EPA regulatory definition of VOC along with the full list of exempt compounds is shown in Figure 2.
EPA is often asked how new compounds are selected for addition to the list of negligibly reactive or exempt compounds. Compounds are usually compared to the reactivity of ethane, which was one of the original four negligibly reactive compounds named in the 1977 policy. Ethane is the most photochemically reactive of the compounds listed in the 1977 list and has, for years, served as EPA's "benchmark" compound. The original experimental work that contributed to the selection of the original four exempt compounds indicated that compounds less reactive than ethane were unlikely to contribute to the national ambient air standard for ozone being violated.2
Figure 2
Definition of Volatile Organic Compounds (VOC) 40 CFR 51.100(s) - Definition - Volatile organic compounds (VOC)
(s) "Volatile organic compounds (VOC)" means any compound of carbon, excluding carbon monoxide, carbon dioxide, carbonic acid, metallic carbides or carbonates, and ammonium carbonate, which participates in atmospheric photochemical reactions.
(1) This includes any such organic compound other than the following, which have been determined to have negligible photochemical reactivity:
- methane
- ethane
- methylene chloride (dichloromethane)
- 1,1,1-trichloroethane (methyl chloroform)
- 1,1,2-trichloro-1,2,2-trifluoroethane (CFC-113)
- trichlorofluoromethane (CFC-11)
- dichlorodifluoromethane (CFC-12)
- chlorodifluoromethane (HCFC-22)
- trifluoromethane (HFC-23)
- 1,2-dichloro 1,1,2,2-tetrafluoroethane (CFC-114)
- chloropentafluoroethane (CFC-115)
- 1,1,1-trifluoro 2,2-dichloroethane (HCFC-123)
- 1,1,1,2-tetrafluoroethane (HFC-134a)
- 1,1-dichloro 1-fluoroethane (HCFC-141b)
- 1-chloro 1,1-difluoroethane (HCFC-142b)
- 2-chloro-1,1,1,2-tetrafluoroethane (HCFC-124)
- pentafluoroethane (HFC-125)
- 1,1,2,2-tetrafluoroethane (HFC-134)
- 1,1,1-trifluoroethane (HFC-143a)
- 1,1-difluoroethane (HFC-152a)
- parachlorobenzotrifluoride (PCBTF)
- cyclic, branched, or linear completely methylated siloxanes
- acetone
- perchloroethylene (tetrachloroethylene)
- 3,3-dichloro-1,1,1,2,2-pentafluoropropane (HCFC-225ca)
- 1,3-dichloro-1,1,2,2,3-pentafluoropropane (HCFC-225cb)
- 1,1,1,2,3,4,4,5,5,5-decafluoropentane (HFC 43-10mee)
- difluoromethane (HFC-32)
- ethylfluoride (HFC-161)
- 1,1,1,3,3,3-hexafluoropropane (HFC-236fa)
- 1,1,2,2,3-pentafluoropropane (HFC-245ca)
- 1,1,2,3,3-pentafluoropropane (HFC-245ea)
- 1,1,1,2,3-pentafluoropropane (HFC-245eb)
- 1,1,1,3,3-pentafluoropropane (HFC-245fa)
- 1,1,1,2,3,3-hexafluoropropane (HFC-236ea)
- 1,1,1,3,3-pentafluorobutane (HFC-365mfc)
- chlorofluoromethane (HCFC-31)
- 1-chloro-1-fluoroethane (HCFC-151a)
- 1,2-dichloro-1,1,2-trifluoroethane (HCFC-123a)
- 1,1,1,2,2,3,3,4,4-nonafluoro-4-methoxy-butane (C4F9OCH3)
- 2-(difluoromethoxymethyl)-1,1,1,2,3,3,3-heptafluoropropane ((CF3)2CFCF2OCH3)
- 1-ethoxy-1,1,2,2,3,3,4,4,4-nonafluorobutane (C4F9OC2H5)
- 2-(ethoxydifluoromethyl)-1,1,1,2,3,3,3-heptafluoropropane ((CF3)2CFCF2OC2H5)
- methyl acetate and perfluorocarbon compounds which fall into these classes:
- (i) cyclic, branched, or linear, completely fluorinated alkanes,
- (ii) cyclic, branched, or linear, completely fluorinated ethers with no unsaturations,
- (iii) cyclic, branched, or linear, completely fluorinated tertiary amines with no unsaturations, and
- (iv) sulfur containing perfluorocarbons with no unsaturations and with sulfur bonds only to carbon and fluorine.
Petitioning for exemption
Many persons have petitioned EPA for additions to the exemptions list. They have argued that if a compound is less reactive than ethane, then it should be considered for VOC exemption. The question remains about exactly how the photochemical reactivity of a compound is compared to that of ethane.There are two ways that have been successfully used in granting VOC exemptions. The oldest and most frequently used method is to compare the reactivity rate constant for the reaction of a compound and the hydroxyl or OH radical with the reactivity rate constant for the reaction of ethane and the hydroxyl radical. The reaction of a compound with the hydroxyl radical is known as the kOH value. The kOH values have units of cm3/molecule-sec.
The reaction rate of a compound with OH or the hydroxyl radical is important because this reaction is the main way in which a compound begins to break down in the atmosphere. If this reaction proceeds rapidly, then other organic radicals will be formed that may further react to form ozone more quickly. If the kOH value is small, the compound will break down slowly and ozone formation will proceed less rapidly.
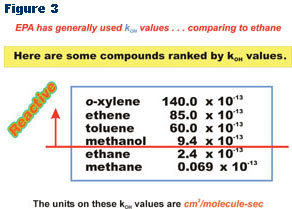
Therefore, if the kOH value of a compound is less than the kOH value for ethane, the compound may be less reactive than ethane and may be declared to be negligibly reactive. Values of kOH have been published in the chemical literature for a wide variety of compounds. (See Figure 3)
Most of the compounds that have been added to the exempt list have been added through a consideration of their kOH values. If the kOH value for a compound is an order of magnitude or lower than the kOH for ethane, then there is strong evidence that the compound is less reactive than ethane. Compounds with kOH values very close to that of ethane may need to be looked at more carefully, since other factors may come into play.
There are nontypical cases that must be considered. For example, there are a few compounds that break down in the atmosphere primarily by photolysis, rather than by the OH radical reaction. For those compounds, additional analysis would be needed to determine if the compound is less reactive than ethane.

Incremental reactivities
The second major method of comparing photochemical reactivity is to compare "incremental reactivities." This is a method that has been studied by several scientists, including Dr. William Carter of the University of California at Riverside.3 The method consists of making a determination of the mechanism by which a compound breaks down in the atmosphere (including, but going beyond the initial reaction with the OH radicals), then applying this information to a computer atmospheric model that is designed to predict the reactivity of the compound.The incremental reactivity is thought by some scientists to be more robust than the kOH method since it takes into account the atmospheric conditions under which the reaction takes place and not just the chemical structure of the molecule under consideration. Very definitely, the other chemicals in the atmosphere in which a compound reacts have an effect on the amount of ozone produced by the compound.
In his work with the state of California, Dr. Carter has studied many different methods of ranking the reactivity of individual VOCs. Dr. Carter concluded that if only one scale could be used for regulatory purposes in California, the maximum incremental reactivity (MIR) scale is the most appropriate (Figure 4>/b>). The MIR predicts the reactivity of a compound at atmospheric conditions where an amount of the organic compound added to the mixture would have the greatest effect on ozone formation. MIR values have units of grams of ozone formed per gram of organic compound reacting.
The MIR values of a compound can be compared to that of ethane to give an indication of whether the compound is less reactive than ethane. The lower the compound's MIR value is below that of ethane, the stronger would be the case that the compound is less reactive than ethane. Dr. Carter has published MIR values for hundreds of compounds on his Web site at the following address: http://pah.cert.ucr.edu/~carter/. See the document related to latest VOC Reactivity Tabulations for SAPRC-99 Mechanism, Appendix C, for a list of MIR values.
A fresh look
A great deal has been learned about reactivity since the 1977 policy was issued 25 years ago. Some people (including the authors) have suggested that perhaps it is time for EPA to review its policy to make sure that it considers the latest scientific thinking. The EPA is taking this suggestion to heart and is currently looking at its policy to see if all or part of it needs revision.There are several practical considerations involved in revising a policy. Any new policy would need to be compatible with ongoing air-quality management requirements that grow out of the federal Clean Air Act. For example, a new policy must be compatible with ongoing SIP control activities, such as emissions-trading programs and new source review (offset) programs. There are practical considerations also, such as the desirability for record keeping to be kept at a reasonable level for compliance and enforcement.
What different possibilities exist for a revised policy? There are many ways to update and/or revise EPA's existing rule. Among the options are:
- Rescind the existing EPA policy and, instead, base the regulation of organic compounds upon the individual ozone air-quality needs of each nonattainment area as determined through the use of photochemical grid models.
- Stay with the existing EPA policy of controlling most organic compounds, but exempting a few compounds with very low reactivity. Develop a standard test protocol, such as the use of a standardized method for measuring kOH values, for reactivity exemptions.
- Continue with essentially the same policy as we have now, but modifying it, perhaps by using a different compound than ethane as the benchmark for exemptions.
- Classify VOCs into groups or "bins" based on their reactivity values. Develop control requirements according to the reactivity value of the bin in which the compound is assigned.
- Assign each compound a reactivity value and use such a reactivity scale to control compounds according to their individual reactivities.
Members include representatives from the chemical, paint and other industries, state regulatory agencies, university researchers and EPA representatives. The current chairman of the RRWG is Dr. Don Fox, who is a professor in the Department of Environmental Sciences and Engineering (School of Public Health) at the University of North Carolina at Chapel Hill. The RRWG is affiliated with NARSTO, formerly known as the North American Research Strategy for Tropospheric Ozone. Information related to the RRWG can be found on the NARSTO Web site at http://odysseus.owt.com/Narsto/reactinfo.html.
The RRWG has approximately three national meetings a year to plan and fund scientific research on reactivity that is relevant to the work of EPA. The primary emphasis is on ozone formation, but fine particulate and haze are also reactivity-related topics that are of interest.
The RRWG has recently funded three atmospheric modeling research projects that look at how changes in the reactivity of emissions will affect ozone levels. The three studies were carried out independently with RRWG funding by three different university and research foundation groups. The EPA plans to examine the findings of these modeling efforts as it reviews its current VOC policy.
Other efforts of the RRWG have included the development of two "white papers" that describe the current state of reactivity science and summarize past and current governmental policy related to reactivity. These two white papers are available on the NARSTO Web site given above.
New regulatory developments
One of the most interesting new regulatory developments related to reactivity is a regulation developed by the California Air Resources Board (CARB) concerning aerosol spray paints. This regulation, which became effective on June 1, 2002, for general spray paints, and on January 1, 2003, for specialty spray paints, attempts to control the product-weighted reactivity of solvents in a coating rather than the total mass of VOC that is emitted.In order to determine compliance with such a rule as this, it is probably necessary to know the identity and weight percent of each of the solvents in the product. First, the MIR value of the solvent is looked up in CARB's table of MIR values. Next, one multiplies each solvent's weight percent by its MIR value and sums the results for all the solvents in an aerosol coating to determine the product-weighted MIR value.
One possible problem with the approach is that considerably more record keeping may be necessary to keep track of the individual solvents in a product. Current mass-based regulations only require that the total mass of VOC be tracked. A record of individual compounds is not needed with a mass-based approach, but is needed with a reactivity-based approach.
The EPA will be looking at how the implementation of the CARB reactivity-based aerosol-coating rule will be carried out in practice. The testing and record keeping aspects required for enforcement will be of great interest. EPA will want to find out if questions of confidentiality of data for the listing of speciated solvents in products pose a problem and/or a regulatory burden upon manufacturers.
>I>Tom Helms is group leader, Ozone Policy and Strategies Group; William Johnson is an environmental engineer, Ozone Policy and Strategies Group; and Stanley Tong is an environmental engineer, Region IX, with the EPA. The Ozone Policy and Strategies Group is part of the Office of Air Quality Planning and Standards (Research Triangle Park, NC).
References:
- Recommended Policy on Control of Volatile Organic Compounds, Federal Register, Vol. 42, No. 131, pages 35314 - 35316, July 8, 1977.
- Basil Dimitriades and S. B. Joshi, Application of Reactivity Criteria in Oxidant-related Emission Control in the USA, International Conference on Photochemical Oxidant Pollution and its Control Proceedings: Volume II, January 1977, EPA-600/3-77-001b.
- William P. L. Carter, Development of Ozone Reactivity Scales for Volatile Organic Compounds, Journal of the Air and Waste Management Association, 44: 881-899, July 1994.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!



