Novel Reactive Hot Melt Polyurethane Adhesives
The chemistry of composite materials is changing at a breakneck pace. The fast-changing world has put more complex requirements on newly introduced products. Each new material advancement and development must not only be better and more favorably priced, but also meet sustainability requirements. So-called composite materials, produced by combining different materials, have played a major role in this development. Among the thermal, mechanical and bonding techniques used to manufacture composite materials, it is anticipated that bonding technology will assume an ever more important role in industry. The bonding technique involves the joining of two substrates using an adhesive.
According to DIN EN 923, an adhesive is defined as: a non-metal; a binder that acts via adhesion and cohesion. Adhesives can be organic or inorganic compounds. Organic and silicone adhesives can be physically hardening (hot melts, wet solvent-containing adhesives, contact, dispersion, water-based, pressure-sensitive adhesives, and plastisols) or chemically curing [polymerization (superglues, methyl methacrylates, unsaturated polyesters, anaerobically curing, radiation curing), polycondensation (phenolic resins, silicones, polyimides, bismalein-imides), and polyaddition (epoxy resins, polyurethanes)].
In 1968, development work began on the moisture-curing polyurethane (PUR) adhesives “Sikaflex” and “Betaseal” for sealing/bonding the front and rear windscreens on cars. And 1970 witnessed the rapid development of polyurethane chemistry with a wide range of 1K and 2K adhesive formulations, including reactive PUR hot melts.
Reactive PUR hot melts are systems (high-molecular-weight, “meltable” polyurethanes with terminal isocyanate groups) that are solid at room temperature and become fairly highly viscous liquids at application temperature of ~120 °C. Typical formulations consist of an isocyanate (such as methylenebis(phenyl isocyanate)), crystalline, amorphous, and/or liquid polyols, moisture-curing catalysts as well as non-reactive additives.1 Each of these components can be appropriately adjusted to obtain the required pot life, open time and green strength. The initial bond strength of reactive PUR hot melts is triggered on cooling by the solidification of non-reactive additives and prepolymers, with the final strength reached via chemical secondary crosslinking by heat, humidity or both.
Although synthetic adhesive technology is almost a century old, researchers continue to advance the technology with an eye on market needs and new regulatory requirements. This article highlights recent advances in adhesive research with special emphasis on polyurethane hot melt adhesives, in particular, polyurethane hot melt adhesives with very fast set rates, resulting in high green bond strength. These new polyurethane hot melt adhesives are designed from conventional polyisocyanates and a mix of proprietary polyols.
Results and Discussion
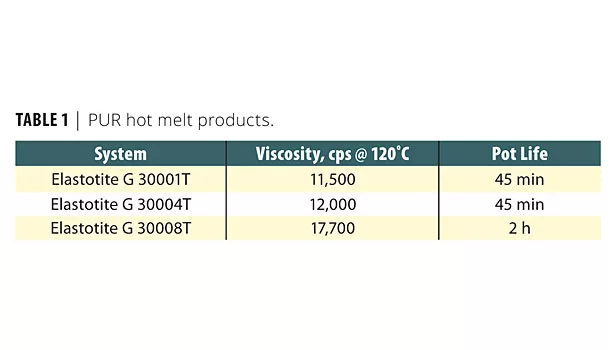
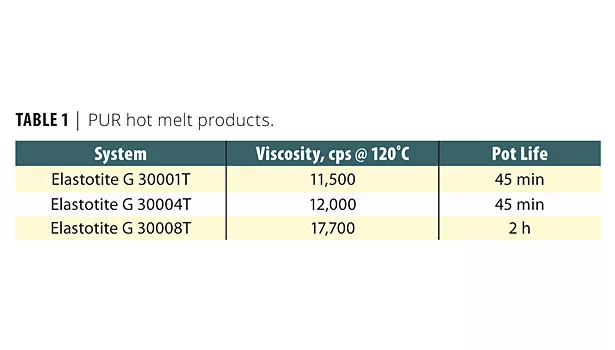
In reactive hot melt polyurethane adhesive formulations, the building blocks, such as isocyanate, polyether, polyester polyols and other proprietary polyols, give each formula its unique characteristics. BASF offers three commercially available PUR hot melts with vastly different physical properties. All three adhesives were successfully designed for three different and unique processes. Some physical characteristics of these adhesives are presented in Table 1.
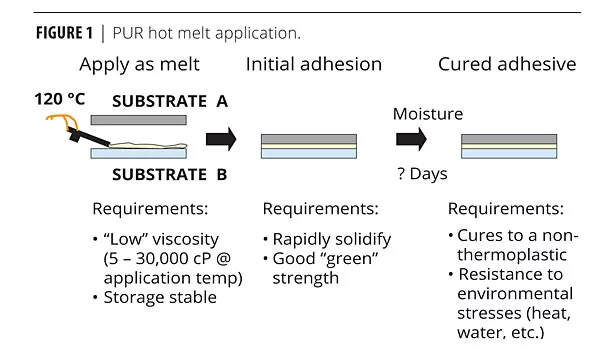
PUR Hot Melt Application
As the name implies, a PUR hot melt must be heated to allow the adhesive to flow. The application temperature can typically range from 110-133 ºC, depending on the formulation and environmental conditions. The application equipment can be either a roll coater or glue gun. Using a roll coater requires the formula to exhibit a residence time. The residence time is the time that a PUR hot melt can be exposed on a roll coater without reacting with the atmospheric moisture. Conversely, the reactivity can be quite fast when using a glue gun since exposure to atmospheric moisture prior to application is minimized. The initial adhesion is the second phase. This is where the PUR hot melt rapidly solidifies and the green strength is invoked. The final cure takes several days, but is 90% complete after the first 24 hours (Figure 1).2
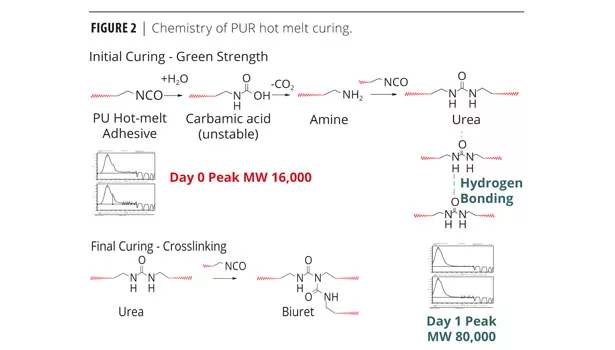
Chemistry of PUR Hot Melt Curing
Figure 2 represents the plausible reaction mechanism from the initial curing to the final thermoset urethane. The sequence of curing begins with water reacting with the NCO-terminated PUR to form the unstable carbamic acid. The carbamic acid quickly liberates CO2, generating an amine. The amine reacts with additional NCO-terminated PUR to form urea. Too much catalyst will cause excess CO2 and a frothy urethane with decreased tensile and tear strength. The carbonyl groups on the urea are attracted to neighboring hydrogen, producing a weak electrical bond, crystallinity and green strength. Finally, the urea may react with the NCO-terminated PUR to form a biuret. The molecular weight increases, providing mechanical adhesion and the final thermoset urethane.3
Green Strength versus Open Time
Green strength is the one property that distinguishes PUR hot melts from all other PUR adhesives. The immediate adhesive strength prior to curing is green strength. It is a cohesive strength that exhibits a resistance to external forces.4 A PUR hot melt’s green strength can be high enough that the substrates do not need to be clamped or mechanically held together. Open time refers to the time that the adhesive remains tacky after application. Generally, the green strength and the open time are diametrically opposed, such that the quicker the PUR hot melt changes from the amorphous liquid state to the crystalline solid the greater the green strength and the shorter the open time.
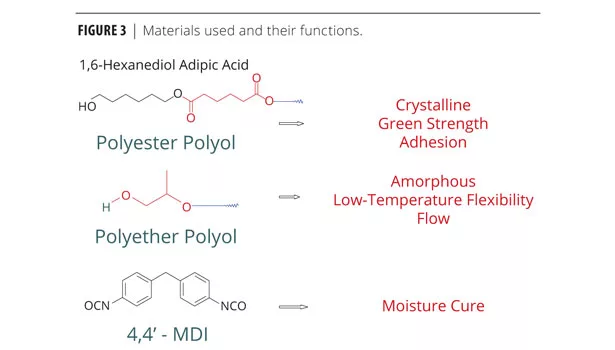
The green strength and open time of the adhesive depend on the morphology of the polymer and the building blocks used to put the molecule together, i.e., the amorphous versus crystalline nature of the PUR hot melt. Some polyester polyols provide the crystalline nature, green strength and adhesion. Polyether polyols typically are amorphous in nature, providing low-temperature flexibility and decreased viscosity, allowing the adhesive to flow. As described earlier, the isocyanate is necessary for the moisture-induced cure (Figure 3).
Using various components in PUR hot melt formulas allows varying degrees of green strength and open time. For example, Elastotite® G 30001T has an open time of minutes, while Elastotite G 30008T solidifies in seconds. The latter adhesive with a short open time allows the composite manufacturer a nonstop production line while maintaining the adhesive’s integrity.
Physical Testing
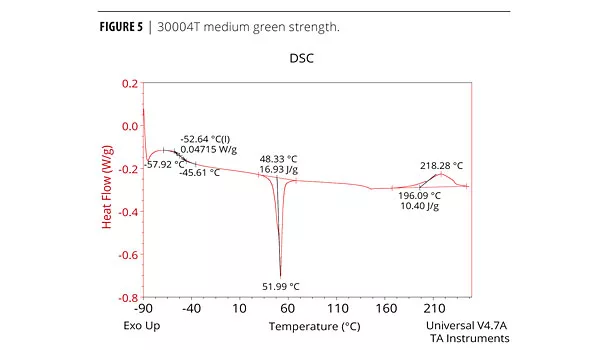
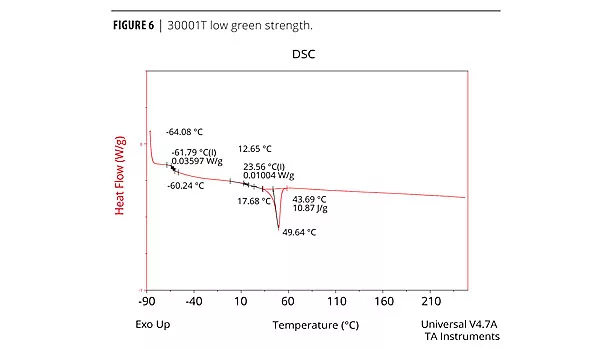
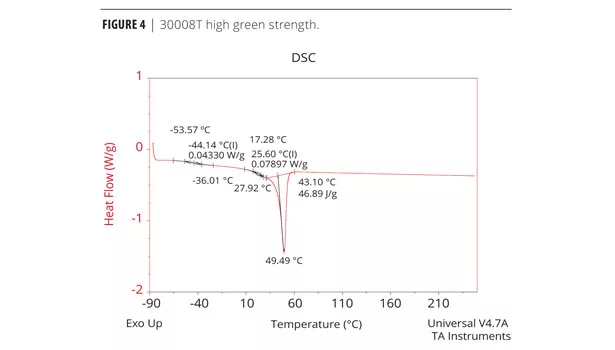
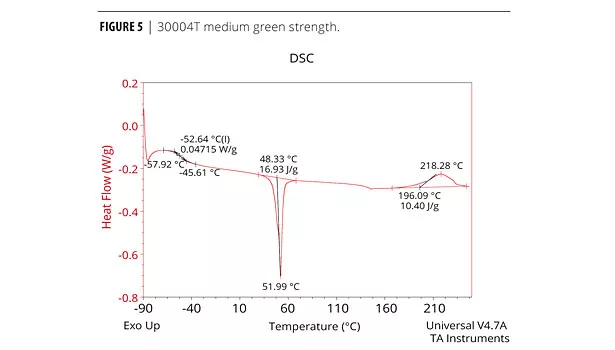
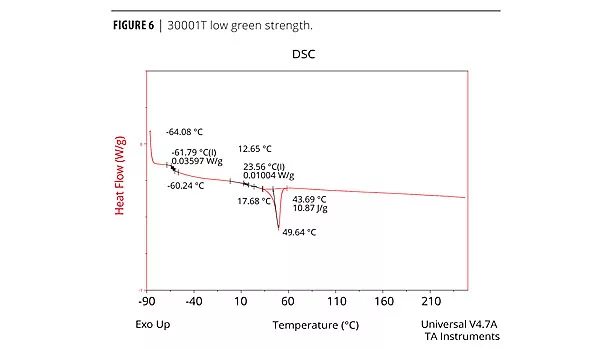
The cure profile resulting in the development of green strength and the final cure properties can be measured analytically and qualitatively. The physical properties (mechanical, thermal etc.) of the adhesive performance are determined by Dynamic Mechanical Analysis (DMA), Differential Scanning Calorimetry (DSC) and Instron for tensile. Both DMA and DSC methods identify the glass transition temperature, Tg, which is the temperature at which the forming urethane phases from the amorphous state to the crystalline state.5 The chemist must determine which method produces the best resolution of a particular formula. Elastotite G 30001, 4, and 8 adhesives with low to high green strength were analyzed by DSC. DSC data is presented in Figures 4, 5 and 6. As expected, all adhesives display thermal events at low and room temperature, with a strong melting endothermic event ~50 °C. In each case, the endothermic melting event probably corresponds to an additive in the formulation and/or solid polyester polyol(s).
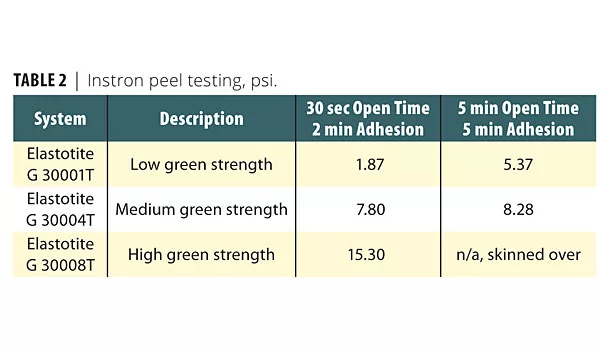
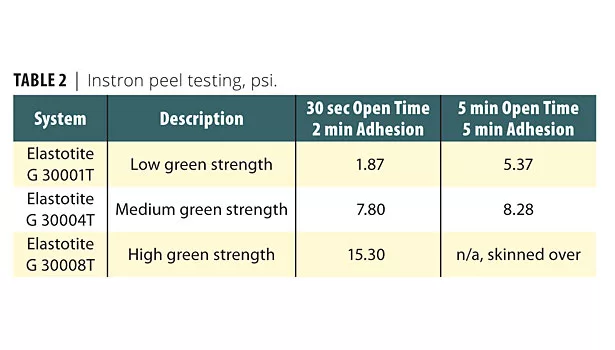
DMA data obtained on all adhesives in this study displayed observations complementary to the DSC data. However, the most useful and astounding performance characteristics of Elastotite adhesives were obtained from the Instron peel testing technique. The data is presented in Table 2. The data shows that Elastotite G 30001T and Elastotite G 30004T adhesive formulations displayed expected performance behavior of the manufactured composite article. However, the Elastotite G 30008T (with BASF proprietary polyols) was novel in that the peel value dramatically improved with 30 sec open time and after 2 min adhesion.
Conclusion
BASF offers a number of Elastotite adhesive products with varying pot life, open time and green strength for various industrial applications. They are manufactured from the reaction products of polyisocyanates and a mixture of proprietary polyols. The Instron peel data for Elastotite G 30001T and Elastotite G 30004T adhesive formulations displayed expected performance behavior of the manufactured composite article. However, the Elastotite G 30008T (with BASF proprietary polyols) was novel in that the peel value dramatically improved.
References
1 Awaja, F.; Gilbert, M.; Kelly, G.; Fox, B.; and Pigram,P.J. Progress in Polymer Science, 34, 948, 2009.
2 Chambers, J.; Moore, T.; Huber, L.; and Frank, D. Fully Reactive PU Hot Melts Offer performance Advantages, Adhesive Age, 24, August 1998.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!