Nanoparticle Additive Accelerates Drying Time of Waterborne Coatings




This paper was presented at the 39th Annual Waterborne Symposium in New Orleans.
The film formation process of one-component waterborne formulations involves a sequence of water evaporation, coalescence and further solidification. The length of this process determines when the coated substrate can be further processed or used. A long drying time lengthens the total coating process until finished parts can be used, further processed or stacked. Accelerating the development of a coating’s early performance characteristics allows for increased productivity if a coating line can run faster, or if the interval between multi-layer coatings can be shortened or if it can extend the application period of a seasonally applied coating.

|
|
Drying time Cytec Resydrol 6150 |
Waterborne coatings have several benefits. Stricter regulations drive the need for lower VOC emissions, and thus the use of waterborne solutions. In addition, waterborne coatings are less hazardous in the application process, are not flammable, and equipment is safely cleaned. Major R&D efforts on waterborne systems have resulted in significant progress and broader availability of waterborne solutions. Today’s customers expect and demand the availability of waterborne products that are perceived as more ‘green’ than solventborne alternatives.
Technically, waterborne coatings are high-tech products that are complex and delicate to formulate. This situation increases the demand for a more comprehensive toolbox to balance the various performance and handling requirements of waterborne coatings. Nanoparticles have been used as functional ingredients in coatings for more than 10 years, even though utilization was sometimes hindered by handling constraints. Aqueous dispersion additives based on inorganic nanoparticles comprise a further advanced class of materials that extend the formulator’s toolbox for waterborne coatings.
Nanoparticle-Based Dispersion Additives
Dispersions of inorganic particles are well established in coatings, e.g., as pigment preparations. From geometrical considerations it is well known that the specific surface area increases drastically (1/x) as the particle size decreases.1 Benefits that rely on the surface area of active ingredients are therefore greatly enhanced when nanoparticles are used. Small amounts of such active ingredients can be very effective, which allows the use of such materials as an additive in the lower percentage or even sub-percentage range. In addition, these materials can be used in clear and glossy coatings, as the visual appearance remains unchanged.
When using nanoparticles, the dispersion quality provides a major hurdle, as the particles need to undergo a threefold change in the environment without agglomeration. First, the actives are dispersed in the carrier liquid. This dispersion needs to be long-term stable. Second, the particles mustn’t agglomerate when introduced into the liquid coating formulation. Here, the in-can stability needs to be maintained and the process parameters shouldn’t be affected too much. Due to the highly complex nature of waterborne coatings, formulations involving many surface-active ingredients such as surfactants, defoamers and leveling agents with various polarities are involved, which is a challenging task. Finally, in the dry film, while water is present in both the carrier liquid and the liquid coating formulation, the particles undergo a final change into a solid and typically hydrophobic phase. It is therefore not only important to provide particles at an excellent particle size distribution but also to modify the particle surface suitably to obtain a useful additive dispersion. At the same time, the surface modification mustn’t passivate the active particle surface, as this would compromise the efficiency of the material altogether.
An economical way to produce suitable nanoparticle dispersions comprises carrying out nanoparticle dispersing and functionalization in one step, i.e., in a chemomechanical process.2 In this process, dispersion and functionalization are carried out in an agitator bead mill. The agglomerated nanopowder is mixed with water and suitable surface-active molecules. The mechanical impact of the grinding beads breaks down the agglomerates. The freshly generated particle surface is immediately covered with the molecules, which prevents re-agglomeration. This process can be continued until ideally the particles are dispersed down to the primary particle size. The starting material and process parameters need to be chosen well for the application to obtain an optimal result.
Such nanoparticle dispersions can be further formulated to obtain a variety of nanoparticle-based additives with increased compatibility in the liquid coating formulation as well as optimized performance in the final film.
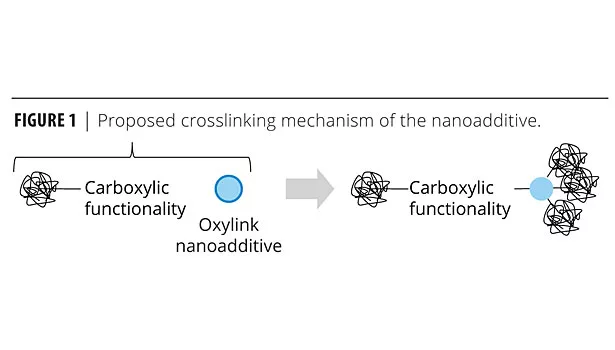
It has been shown previously that Oxylink additive increases the resistance against chemical stress such as humidity, solvents, weathering, as well as blocking resistance.3-5 The effects are based on increased crosslinking in the final film due to interaction of the particles with carboxylic groups of the polymer. Figure 1 illustrates a possible mechanism. We have also noted that using this additive reduces the drying time, and we investigate this effect in more detail in this article.
Accelerating Drying Time
During the drying process, the film’s resistance properties increase steadily, as determined by drying time recorder, pendulum hardness, blocking characteristics or water resistance tests. When the crosslinking occurs sufficiently fast, it can increase the resistance properties during the drying process, resulting not only in higher final resistance properties but also attaining a given film toughness at an earlier time (Figure 2). The suggested crosslinking mechanism involves only non-covalent interaction, which can happen quickly, as soon as water evaporates. In contrast, it doesn’t involve the formation of covalent bonds and major molecular rearrangement, which slows down chemical crosslinking in a polymeric high-viscosity environment such as a drying coating.
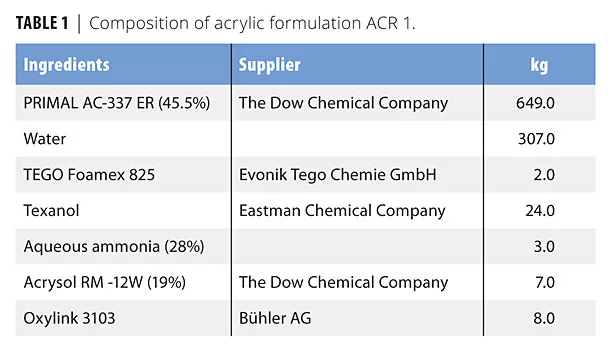
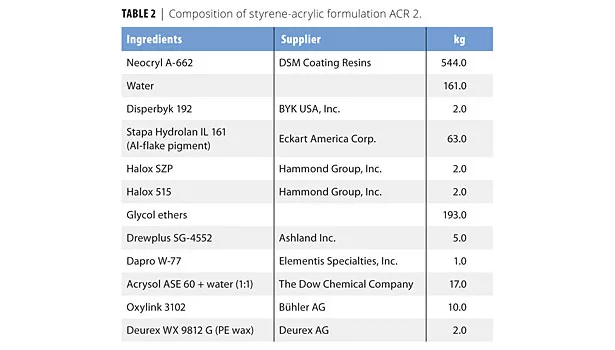
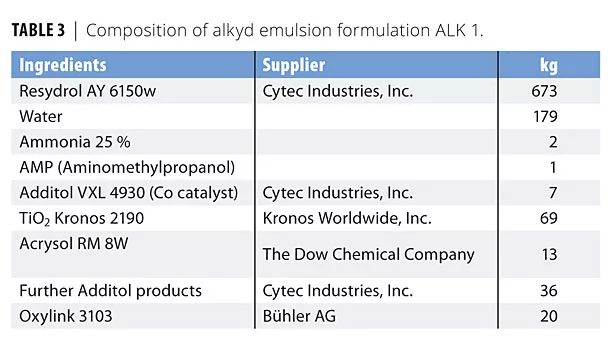
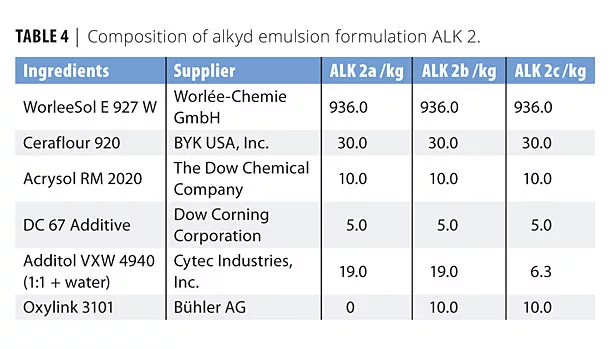
In this study we evaluated four formulations based on two acrylic (ACR 1 and 2) and two alkyd (ALK 1 and 2) emulsions. The formulations were chosen to cover different areas of application. Tables 1-4 list the compositions used in this study. The formulations are also available at www.buhlergroup.com/oxylink.
Experiments and Results
The drying time for ACR 1 to drying level 4 was measured according to DIN 53150. We coated glass slides with a 200 µm wet film and determined the duration until drying level 1 (glass pearls do not stick on coating) and 4 (a paper that is pressed by a 2 kg weight to the coating does not stick) were reached. Figure 3 illustrates the results.
The blocking score for ACR 2 over time was determined to characterize the time after which finished parts can be stacked without damage.6,7 We coated Leneta® foils with a 60 µm wet film and determined the blocking score according to Richtlinie 6, IFT Rosenheim on a 5 point scale (0: best, 5: worst) over time.6 Figure 4 illustrates the results.
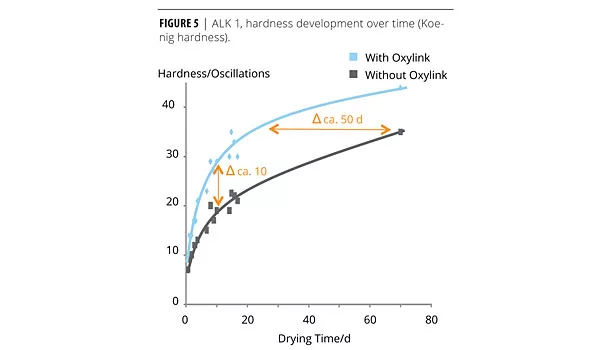
We coated glass slides with ALK 1 (100 µm wet film) and followed the drying characteristic of the film using Koenig hardness measurements over 70 days. Figure 5 illustrates the development of pendulum hardness over time.
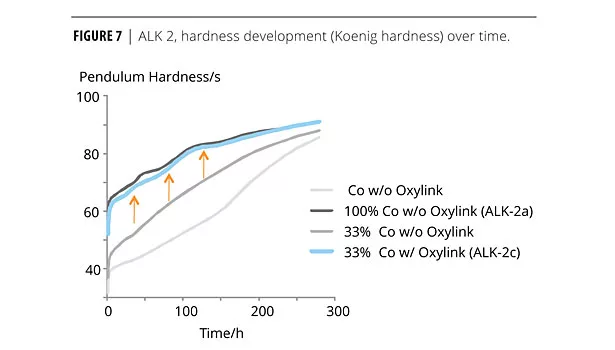
We also coated glass slides with ALK 2 (100 µm wet film) and followed the drying characteristic of the film using Koenig hardness measurements over 12 days (ALK 2a vs. ALK 2b) (Figure 6). In another experiment, we reduced the content of oxidative (cobalt) drier in the formulation (ALK 2a vs. ALK 2c). Figure 7 illustrates the development of pendulum hardness over time.
Discussion
The drying time reduction for ACR 1 was reported earlier, along with the strong crosslinking effect as determined by MEK rub resistance tests.3,8 ACR 1 was a formulation of a clear, high-build wood stain based on a 100% acrylic emulsion. Drying grade 1 remained unchanged at 30 min. However, while without the additive drying grade 4 wasn’t reached even after 48 h, 1% of the additive resulted in a drying grade 4 after only 20 h. The effective crosslinking of this resin results in a number of further benefits such as increased resistance against organic solvents and increased blocking resistance. Studies on anti-blocking agents have shown that blocking is mainly influenced by limiting the migration of small molecules (see Reference 9 for a more detailed discussion). Crosslinking polymer chains and/or small molecules can therefore effectively reduce the blocking effect. For ACR 1, the blocking score was improved from 5 (worst value) to 1 in 24 h.
Blocking is the main interest for ACR 2.7 The formulation was a plastic coating based on a styrene-acrylic resin. It contained aluminum flake pigments to give the coating a finish desired for products such as stereo equipment and automotive interiors. We evaluated the time after which a sufficiently low blocking score was achieved so freshly painted parts could be stacked. The optimized formulation ACR 2, which included 1% of the additive, reached a suitable blocking score of 1 after only 2 h, whereas the same system without Oxylink reached the same score only after 3 h. This reduction of relevant dry time by 33% allows a faster turnover of finished parts when used in an OEM environment. Interestingly, the optimized formula also contained a small amount of a polyethylene wax (0.2% Deurex WX 9812). We have found a synergistic effect with this wax and the nanoadditive Oxylink: without the inorganic nanoadditive, even larger amounts of wax don’t reduce the blocking score, and vice versa. While this effect isn’t understood in detail, it shows the importance of optimizing the final formulation.
Alkyd emulsions have been developed to combine the excellent resistance and appearance of alkyd resins with waterborne technology. Formulation ALK 1 was a thick-film, white pigmented formulation for wood protection based on an acrylic-modified water-based alkyd emulsion. The thick-film application makes it suitable for challenging environments such as yacht coatings, however it poses a challenge to the drying time as determined by pendulum hardness. Without a nanoadditive the hardness developed very slowly, and even after 2 months only ca. 30 oscillations are found. To reduce the development cycle, we first evaluated increasing concentrations of Oxylink on drying time using a drying time recorder over 12 h. While the drying time as determined by drying time recorder was traced over a much shorter period of time, it is useful to establish the right concentration of Oxylink. The effect of the nanoadditive on the drying time is clearly seen in the trace when using 1.5% on total formula, and 2% of the additive shortened the trace considerably. We therefore chose a concentration of 2% to monitor the hardness development in the more time-consuming pendulum hardness determination. With Oxylink, the pendulum hardness lies consistently ca. 10 oscillations above the hardness of the same formulation without the additive (Figure 5). Due to the kinetics of the hardness development, this is equivalent to an acceleration of ca. 50 days.
Formulation ALK 2 was a clear, matte formulated wood coating based on a short-oil, PU-modified alkyd emulsion. With 1% Oxylink, the pendulum hardness lies consistently ca. 5 oscillations above the hardness of the same formulation without the additive. As a result we found the same pendulum hardness 14 days earlier when using the nanoadditive. Even though the ZnO-based nanoadditive accelerated the hardness development, it doesn’t substitute as an oxidative dryer in the formulation. When formulating an alkyd emulsion without cobalt, adding Oxylink basically had no effect on the drying time. Instead, the nanoadditive showed a synergistic effect in combination with the cobalt component. It is therefore possible to greatly reduce the amount of cobalt drier in ALK 2 while maintaining the hardness development. Figure 7 illustrates this optimization: without cobalt drier, pendulum hardness still developed, but very slowly. After 150 h (ca. 1 week), the hardness was still below 60 sec, while the full amount of oxidative drier (ALK 2a) resulted in a hardness of 85 sec after the same drying time. Reducing the Co drier to 1/3 of the original amount resulted in a hardness development between those two: after 150 h, a hardness of ca. 70 sec was achieved. However, adding the nanoadditive to this composition restored the original hardness development: formula ALK 2c contained only 1/3 of the cobalt amount of the comparative ALK 2a, but had the same hardness development characteristics due to an additional 1% Oxylink in the formulation. We suggest that this effect offers another benefit in the effort to formulate greener coatings.
Summary
We compared the drying of various waterborne formulations for different applications. In all evaluated formulations, a specific film stability was reached after a significantly shorter time when a nanoparticle-based additive was added. The addition of just 1-2% of additive on total formula was effective. n
References
1 Pilotek, S.; Schär, S.; Steingröver, K.; Gossmann, K.; Tabellion, F. NSTI-Nanotech 2007, Vol. 2, 64 2007.
2 Homann, H.; Näf, H.; Pilotek, S.; Tabellion, F.; Steingröver, K. Proceedings on International Congress on Particle Technology, Nürnberg (Germany), March 27-29, 2007.
3 Burgard, D.; Herold, M.; Steingröver, K. Asia Pacific Coating Journal, Aug. 2009, 26-28.
4 Pilotek, S.; Burgard, D.; Herold, M.; Steingröver, K. The Waterborne Symposium, New Orleans, Feb. 18-20, 2009.
5 Pilotek, S.; Burgard, D.; Steingröver, K.; Herold, M. Third International Coating Wood and Wood Composites Conference, Charlotte, NC, Sep. 22/23, 2009.
6 Rosenheimer Richtline R6, Verblockung von Anstrichsystemen auf Holzfenstern, 3/99.
7 Burgard, D.; Herold, M.; Steingröver, K. Asia Pacific Coating Journal, Oct./Nov. 2010, 22-23.
8 Burgard, D.; Herold, M.; Steingröver, K. European Coatings Journal, 11, 2009, 22-26.
9 Burgard, D.; Herold, M.; Steingröver, K. Farbe und Lack, 117 (6), 2011, 14-18.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!




