Improved Saturated Polyester and Alkyd Coatings
From Renewable, Bio-Based Building Blocks





One of the major drivers for innovation in the chemical industry today is sustainability. This not only relates to marketing claims, but will be a general trend in the industry for many years. Terms like ‘carbon footprint’, ‘independent of fossil resource-based raw materials’, ‘cradle-to-cradle design’ and ‘sustainable production’ are used more frequently and are increasingly incorporated into mainstream industry. This momentum toward sustainability can be used in such a way that a change in formulation also improves efficiency and product performance.
A sustainable business can be depended upon by its customers to supply products now and in the future. The megatrend toward making industry more sustainable has been translated by the coatings industry and legislative bodies into a number of (measurable) targets, such as CO2 (carbon) footprint and VOCs.
One way to achieve these targets is to incorporate renewable raw materials into coatings. These raw materials should not only contribute to a reduction of the CO2 footprint over the life cycle of the coating, but should also be cost-efficient and provide equal, or better, coating performance than petrochemical alternatives.
Purac has introduced its renewable PURALACT™ lactide as a monomer for creating sustainable coating resins. This bio-based building block provides the desired CO2 reduction, is available on an industrial scale and offers the possibility to enhance coating performance.
Lactide-Modified Resins
Lactide can be used in a wide range of applications, such as resins for coatings and elastomers. The resins presented in this article demonstrate the use of lactide in saturated polyester and alkyd resins for coating applications. The research goal was to incorporate certain levels of lactide to improve the carbon footprint of the final coating, while maintaining the final properties, compared to the reference formulation, as well as keeping the resin formulation cost-neutral.
Saturated polyester resins are of increasing importance in coatings, as demands for greater hardness, flexibility, impact strength and chemical resistance intensify. These saturated polyesters have major importance in paints and lacquers.
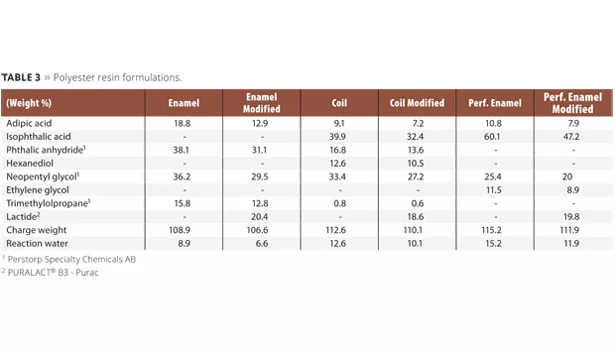
In total, three saturated polyester resins for three different types of industrial applications, namely enamel, coil and performance enamel coatings, were chosen to demonstrate the use of lactide. These polyester resin formulations were modified with 20% lactide by replacing a mixture of di-functional glycol and diacids (Table 1). The overall formulation was adjusted to maintain the properties of the reference formulation. The carbon footprint of the final resin was based on the Purac Lactide Life Cycle Analysis,1 and depended upon the data from the ecoinvent Centre and our raw material suppliers. The incorporation of lactide improved the carbon footprint by 18-27% (Figure 1).
Alkyd resins still offer a very attractive solution for meeting the environmental challenges that the paint industry is facing. The continuing demand to lower VOCs and reduce carbon has forced producers to reformulate their resins to alkyd emulsions or high-solid systems. Alkyd resins are still favored for their gloss, flow and leveling properties.
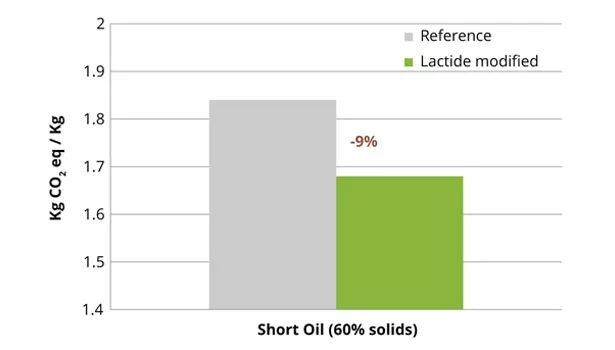
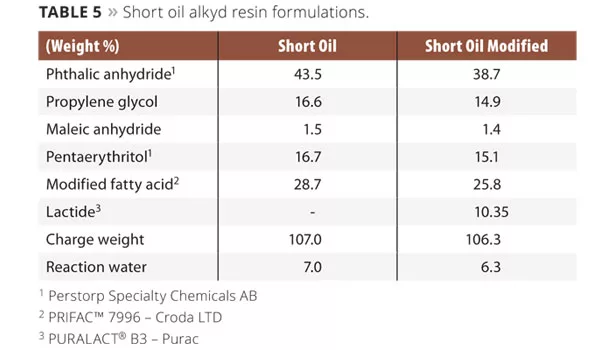
In Purac’s research, one non-air drying (short oil) industrial alkyd resin for two coating applications (enamel and 2K PU) was chosen to demonstrate the use of lactide. The alkyd resin formulation was modified with 10% lactide by replacing a mixture of di-functional glycol and diacids (Table 2). Again, the overall formula was adjusted to maintain the properties of the reference formulation. Similar to the polyester resin, the incorporation of lactide improved the carbon footprint of the alkyd resin, based upon the carbon footprint of the raw materials, by 9% (Figure 2).
Experimental
Polyester Resin Synthesis
Polyester Formulation for Enamel Coatings
The polyester resin for enamel coatings was synthesized using xylene as an azeotropic solvent. All raw materials (Table 3), including xylene (3-5% on resin), were added to the reactor. The reactor was equipped with a thermometer, inert gas purge, agitator, intermediate cooler (95-100 °C) and condenser. Heating was performed under gas purge (in 3 h, to 210 °C), and agitation was started when possible. The reaction temperature was maintained until an acid value of 10 mg KOH/g was obtained. The reactor was cooled, and the final resin diluted to 75% solids in xylene.
Polyester Formulation for Coil Coatings
All raw materials (Table 3) were charged to the reactor equipped with a thermometer, inert gas purge, agitator and condenser. Heating was performed under gas purge (in 2 h, to 200 °C), and the agitation was started when possible. Heating was continued to a maximum reaction temperature of 225 °C, while maintaining an overhead temperature of 100 °C. The reaction temperature was maintained until an acid value of 10 mg KOH/g was obtained. The reactor was cooled, and the final resin was diluted to 60% solids in propylene glycol methyl ether acetate (PMA)/Solvesso 150 (ratio 3:1).
Polyester Formulation for Performance Enamels
The polyester resin for performance enamel coatings was synthesized in a two-step process, using xylene as an azeotropic solvent. Neopentyl glycol, ethylene glycol and isophthalic acid (Table 3, lactide optional), including xylene (3-5% on resin), were added to the reactor. The reactor was equipped with a thermometer, inert gas purge, agitator, intermediate cooler (95-100 °C) and condenser. Heating was performed under gas purge (12 °C/h to 175 °C, then 6 °C/h to 210 °C), and agitation was started when possible. The reaction temperature was maintained until a clear solution was obtained. The system was cooled to 170 °C, and trimethylolpropane and adipic acid (Table 3) added to the reactor. The temperature was raised again to 210 °C and maintained until an acid value of 10 mg KOH/g was obtained. The reactor was cooled and the final resin was diluted to 70% solids in xylene/methoxypropanol acetate (ratio 4:1).
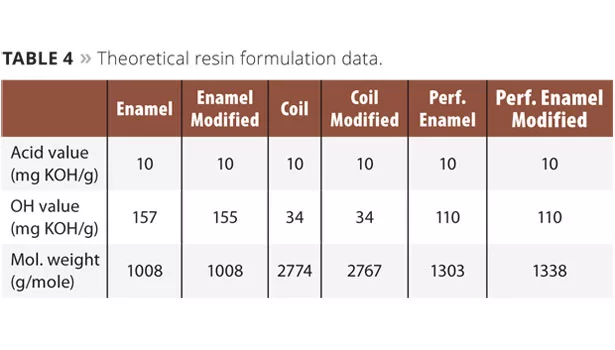
The theoretical resin data for all formulations is presented in Table 4.
Alkyd Resin Synthesis
Short Oil Alkyd Formulation
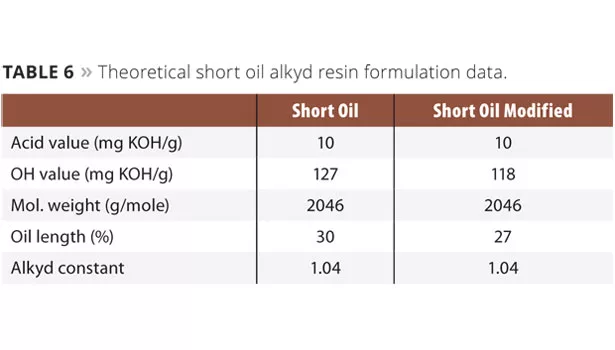
The short oil industrial alkyd resin was synthesized using xylene as an azeotropic solvent. In the case of the short oil alkyd resin, the phthalic anhydride and propylene glycol (Table 5), including xylene (3-5% on resin), were added to the reactor. The reactor was equipped with a thermometer, inert gas purge, agitator, intermediate cooler (95-100 °C) and condenser. Heating was performed under gas purge (to 180 °C) and the agitation was started when possible. The reaction temperature was maintained until an acid value of 10 mg KOH/g was obtained. The system was cooled to 120 °C, and the maleic anhydride, pentaerythritol (Table 5, lactide optional) were added to the reactor. The temperature was raised to 230 °C and maintained until an acid value of 10 mg KOH/g was obtained. The reactor was cooled and the final resin was diluted to 60% solids in xylene. The theoretical resin data for all formulations can be obtained in Table 6.
Coating Formulations
Polyester Coating Formulation
All industrial polyester resins were formulated as clearcoats. The solids content of the polyester resins, as well as the ratio between resin and hardener, differed depending on the application of the coating (Table 7). The coatings were applied on different substrates, depending on the evaluations, using a 50 µ wire bar. Curing was performed in an oven for 15 min at 160 °C.
Alkyd Coating Formulation
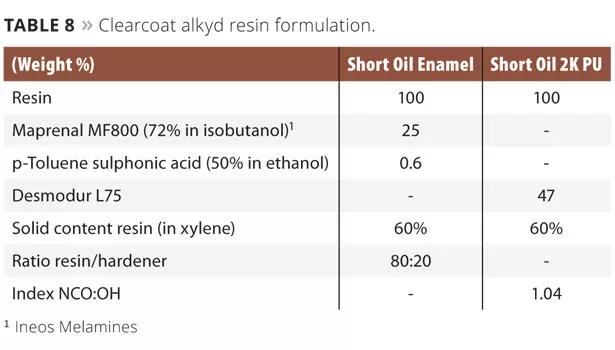
The alkyd resins were formulated as clearcoats (Table 8). The coatings were applied on different substrates, depending on the type of evaluations, using a 100 µ wire bar. Curing of the short oil enamel alkyd-based coatings was performed in an oven for 30 min at 150 °C, while the short oil 2K PU alkyd-based coating was cured at room temperature.
All clearcoat formulations were evaluated for their mechanical properties and chemical resistance on different substrates. The mechanical coating evaluations consist of König (ISO 1522) and pencil hardness (ISO 15184), conical bend (ISO 6860), adhesion (cross-cut, ISO 2409), surface tension, and direct and indirect impact strength (ISO 6272-1). Furthermore, the coating formulations were evaluated on chemical and stain resistance (ISO 2812 and DIN 68861). The lactide-modified enamel polyester resin coating was also evaluated to assess its outdoor stability (ISO 4892-2A). The alkyd resin coating formulations were evaluated after forced ageing and gloss (20°, 60° and/or 85°).
Results and Discussion
Resin Synthesis Results
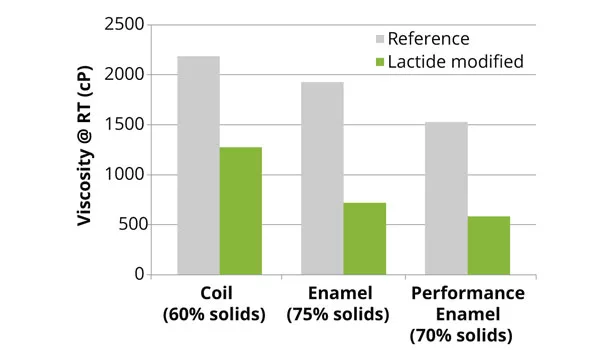
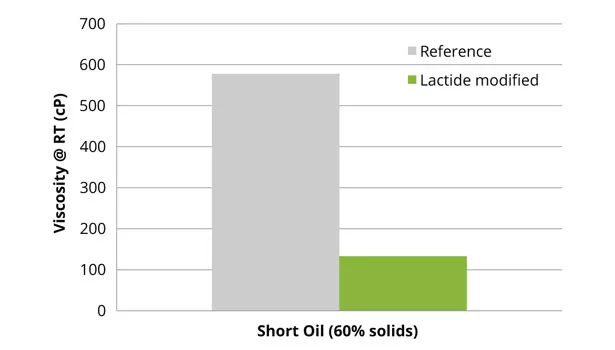
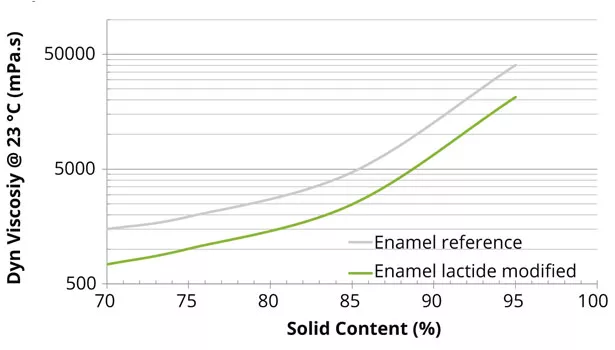
The reference and lactide-modified polyester and alkyd resins were prepared according to the above-described methods. The final properties of the enamel, coil and performance-enamel resins, as well as the short oil alkyd resin, were similar to the theoretical data presented. The amount of reaction water of the lactide-modified resins was 10-25% lower than the reference system. This reduction in water, caused by the ring-opening polymerization of lactide, will be beneficial in solventborne coatings, since less water needs to be removed from the final resin. The lactide-modified polyester resins all showed a decrease in viscosity compared to the reference resins (Figures 3 and 4).
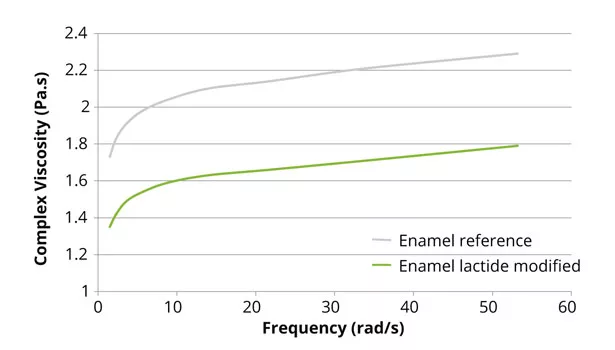
The reduction in viscosity of the lactide-modified resins also enables higher-solid formulations, leading to lower VOC content in the final coating (Figure 5), since the working viscosity of the coating can be maintained. Lactide not only lowers the viscosity of the final resin, but modification with lactide also retains the rheological behavior of the polymer resin (Figure 6).
Coatings Formulation Results
Polyester Coating
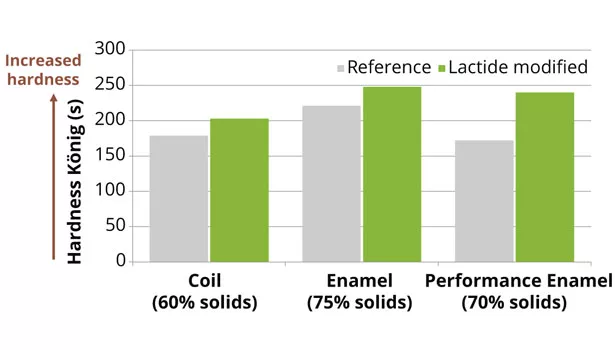
After curing, the lactide-modified polyester coatings show enhanced hardness, both pencil and König (Figure 7), while simultaneously maintaining the coating flexibility in conical bend evaluations (Figure 8).
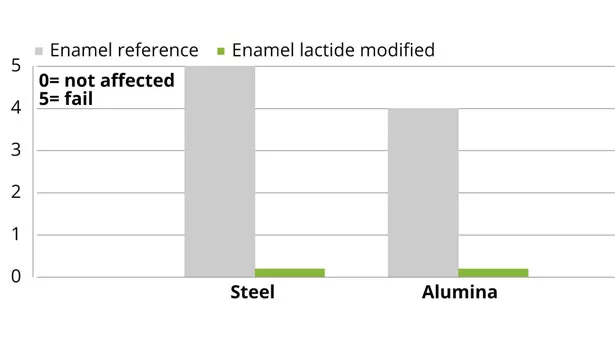
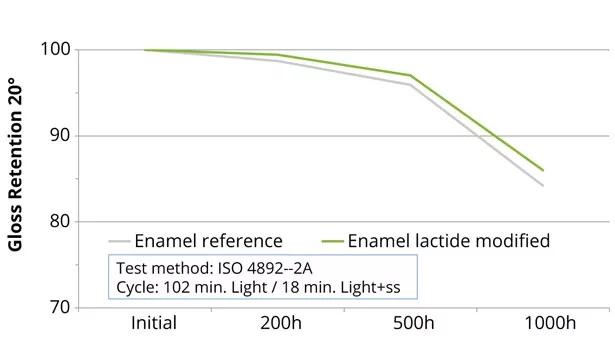
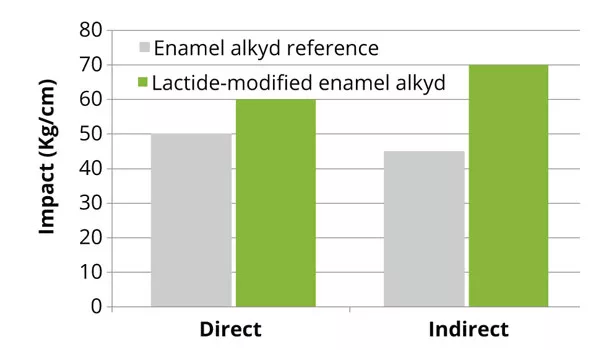
Impact resistance of lactide-modified polyester coatings, as well as adhesion on metal substrates for coil- and performance-enamel coatings, were maintained compared to the reference coatings. Adhesion on metal substrates for the enamel polyester coating was significantly improved, versus reference (Figure 9). Adhesion was improved because of the more polar structure of the polymer created by the incorporation of lactide. The chemical and stain resistance for all lactide-modified polyester coatings showed comparable results. Furthermore, modification of the enamel coating with lactide exhibited a comparable outdoor behavior compared to the reference coating (Figure 10). The outdoor stability of the enamel coating during 1000 h is comparable to exposure to the Northern European climate for 1 year.
Alkyd Coating Formulation
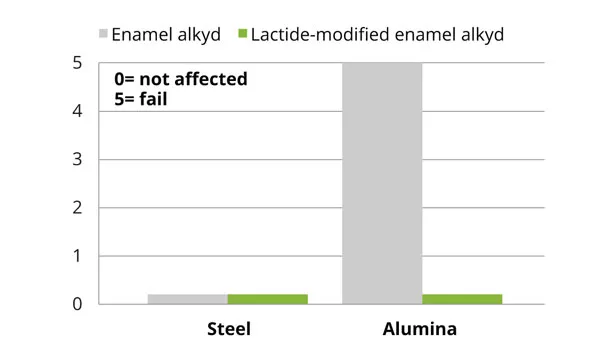
The lactide-modified enamel industrial alkyd coatings (short oil) showed an improvement in flexibility compared to the reference coating (Figure 11). This improved flexibility is caused by introducing a smaller, more flexible monomer. The hardness of both the lactide-modified enamel and 2K PU alkyd coatings was comparable to the reference. The adhesion on aluminium substrates for the enamel alkyd coating was significantly improved versus the reference (Figure 12), while the 2K PU alkyd coating showed similar results to the reference. The reference and lactide-modified industrial coatings exhibited comparable chemical and stain resistance, as well as similar discoloration, under forced conditions.
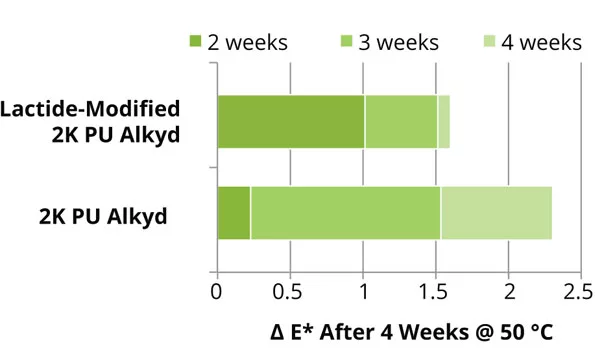
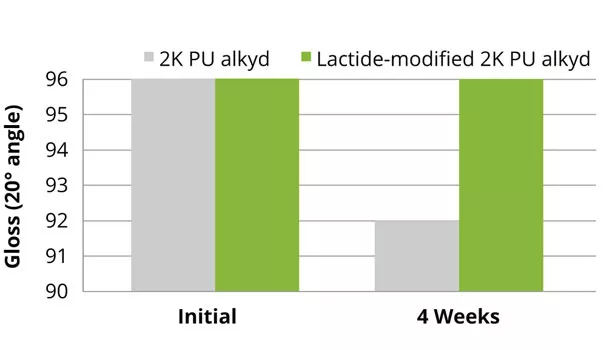
Both after curing and storage under forced conditions, the lactide-modified enamel alkyd coatings showed similar gloss and yellowness index compared to the reference alkyd coatings. The results of the color development (4 weeks in the dark, at 50 °C after 1 week drying at room temperature) and gloss of the 2K PU alkyd coatings are shown in Figures 13 and 14. The lactide-modified coating shows an improvement of the discoloration and improvement in gloss after storage under forced conditions. The reference 2K PU alkyd coatings show similar initial gloss, but deterioration of the gloss after storage under forced conditions.
Conclusion
Lactide provides resin producers with a versatile, commercially available, bio-based building block for saturated polyester and alkyd resins for both industrial and architectural coating applications. It is possible to reformulate reference resin formulations with lactide by replacing oil-based monomers, such as di-functional glycols and di-acids. The carbon footprint of the resins can be improved by up to 27%, depending on the carbon footprint of the raw materials and formulation. The renewable content of the total formulation can also be improved.
Furthermore, lactide-modified resins exhibit lower resin viscosity, but retain the rheological behavior of the polymer resin. This introduces the possibility of producing high-solid formulations. With current demands for lower organic solvent emissions, the incorporation of lactide into the resin system will enable resin producers to meet these new targets.
Lactide-modified polyester resins enhance the hardness of industrial coatings, while maintaining flexibility. In the case of the enamel polyester coating, flexibility, as well as adhesion on metal substrates, are improved by incorporating lactide. Finally, modification with lactide exhibits comparable outdoor behavior compared to the reference coating, indicating that outdoor stability is not affected by incorporating lactide.
The enamel alkyd coatings containing lactide-modified short oil resins show an improvement in flexibility due to introduction of a smaller, more flexible monomer, while maintaining hardness. The adhesion of this alkyd coating was especially improved on alumina. The lactide-modified 2K PU coatings show an improvement in the discoloration and gloss retention under forced conditions.
To conclude, lactide is a versatile, bio-based building block that can be easily incorporated into saturated polyester and alkyd resins for industrial coating applications; lactide improves sustainability of the coating, as well as enhances performance of both resin and coating. n
References
- Groot W.J.; Borén T. (2010) Life cycle assessment of the manufacture of Lactide and PLA biopolymers from sugarcane in Thailand; The International Journal of Life Cycle Assessment, November 2010, Volume 15, Issue 9, pp 970-984.
For more information, e-mail: bas.van.leeuwen@purac.com or visit us during the European Coatings Show (Hall 1, Stand 1-346).
Presented at the 2013 European Coatings Conference.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!





