Exploring Next-Generation Methods in TiO2 Optimization

One of a paint formulator’s constant concerns is to find ways to reduce titanium dioxide (TiO2) content in formulations without compromising key paint properties. Due to increasing TiO2 consumption worldwide and unchanged production capacities in the United States and in Western Europe, TiO2 prices increased recently by more than 50%. Such fluctuations are difficult to manage for paint manufacturers.
Facing the Situation
Paint manufacturers have adapted to the price volatility in several ways. Using cheaper TiO2 grades is one of them, but, for general market shortages, price differences tend to be less for TiO2 obtained from a similar technology. Some cost saving can be achieved by considering TiO2 obtained from another technology and, as a result, more buyers are increasingly interested in TiO2 produced in Asia, and more specifically in China.
Most of the TiO2 grades produced in Asia are obtained from the sulphate process, which is considered to generate TiO2 of lower quality and is more difficult to formulate with than TiO2 obtained from the chloride process, which is mostly produced in the United States and Western Europe. Additionally, the sulphate process is known to generate more waste, while consuming more water. For environmental reasons, the Chinese government recently affirmed its commitment to restrict the sulphate process, thus benefiting the chloride process, for future investments. In addition to the environmental concern, the Chinese government’s aim is to make China a first-rank supplier for high-quality TiO2 grades that are able to compete with the grades produced in Western Europe and the United States.
Any strategy to reduce cost by using cheaper TiO2 grades may only be a short-term approach; it does not prevent intensive laboratory work and there is no guarantee that the necessary and expected results will be achieved. Furthermore, U.S. TiO2 suppliers have invested substantial resources in designing high-quality, stable and easy-to-use TiO2 slurries, which are highly correlated to the quality of the TiO2 itself. Using slurries made of cheaper TiO2 grades in the United States or Western Europe would require asking Asian manufacturers to design slurries stable enough to withstand long oceanic transportation times of several weeks and months of storage – neither of which is simple or realistic, or for the paint manufacturers to employ designing and making the mineral slurries themselves, thus resulting in significant development and process investments.
Using Less TiO2
A long-term strategic and environmentally sustainable approach is to reduce the need for TiO2. A way of lowering TiO2 amounts was investigated many years ago by using specific organic polymers (e.g., hollow sphere).
Reformulation with such polymers involves changing both the binder and TiO2. In order to achieve the maximum benefit from the change, paint manufacturers often must rely on the reformulation proposals made by organic pigment suppliers that generally also highlight synergistic effects with binders from the same supplier. Despite its success, the approach limits the flexibility of formulation and implies that the formulation already incorporates a significant amount of binder.
Most paint manufacturers are now looking for the next milestone, i.e., saving more TiO2 than previously achieved and extending the savings options to all types of formulations, even the ones showing a lower amount of binder. More innovative ways of saving TiO2 are therefore required that at the same time are less binder dependent and more focused on the intrinsic valorization of TiO2 particles. High TiO2 valorization simply sets the following target: to achieve the best dispersion and stabilization of TiO2 particles in the water phase, both in diluted and in the highest concentration conditions. Highest concentration conditions are related to the latter stages of the drying process of the paint film, while diluted conditions are related to the mill base or slurry making, as well as to the bulk stability over time.
Dispersants Need Formulators’ Attention
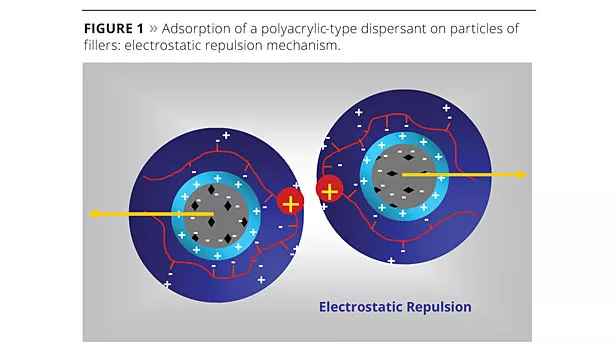
The dispersion and stabilization of TiO2 in dilute conditions depends mainly on the use of well-adapted dispersants. Dispersants are required when significant amounts of fine fillers or pigment particles are added to water to make the mill base. Deflocculation is needed because powdered pigment particles tend to agglomerate during storage in bulk or big bags due to the combined compression and remaining moisture. The added dispersant plays the dual role of lubricant and dissociative agent and, once the particles are de-agglomerated, it interacts with their surface by forming a protective organic layer. Once wrapped around the particle, the dispersant contributes to build an additional layer made of positive charges (Figure 1) that oppose the attraction tendency between the particles.
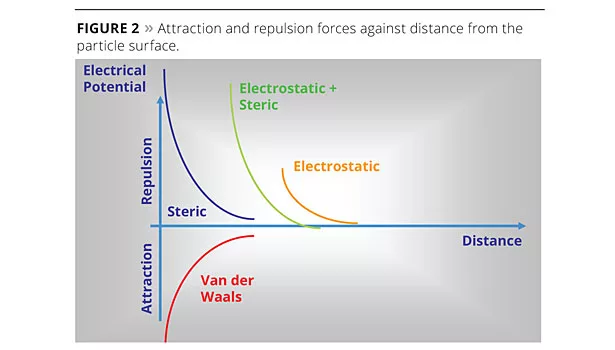
Stability of the particle suspension is ensured by both the re-enforcement of the electrostatic repulsion potential, thanks to the double ionic layer and by the steric hindrance effect (Figure 2).
When playing on the ionic mechanisms is more difficult or even not possible, the use of nonionic dispersants should be considered. Dispersants adapted for the dispersion of mineral particles in water and showing a reduced ionic character offer new potential and insight for formulators. Instead of wrapping the surface of the mineral particles, non-ionic dispersants ensure their spacing due to a comb structure based on long ethoxylated moieties. This way of stabilization can be adapted for titanium dioxide or extenders such as precipitated or natural ultrafine calcium carbonate. Stabilization by spacing also brings advantages for the dispersion of nano-sized mineral particles and gives more flexibility regarding the pH adjustment.
Selecting the Best Adapted Dispersants
Acrylic-based dispersants are shown to provide decisive advantages in terms of effectiveness, stability and water resistance and, therefore, often replace the older generation of phosphate-based dispersants: tripolyphosphates (TPP), pyrophosphates or hexametaphosphates (HMP). In particular, acrylic-based dispersants offer a long-lasting stabilization effect when the paint is stored at temperatures higher than room temperature. This is not the case with polyphosphate dispersants, which suffer from degradation by hydrolysis under the same conditions.
When it is necessary to disperse specific or very fine fillers and pigments such as TiO2, the use of dispersants based on a co-polymeric structure must be considered. Acrylic-based monomers can be combined with esters or monomers showing a hydrophobic character. Side chains bearing hydrophobic end groups can also be grafted to interact in specific ways with hydrophobically modified pigment or binder particles. They can also contribute to an increased hydrophobic character of freshly applied coatings (early rain resistance) and of dried coatings (scrub resistance).
How to Optimize TiO2 Dispersion
One way to optimize the use of TiO2 is to ensure its proper dispersion in water when making the mill base. A powerful stirrer should be used and enough time allowed (at least 20 min). The dispersant system should also contribute to better TiO2 deflocculation and stabilization.
Therefore, replacing so called “universal” additives is highly recommended, as performance of TiO2 is often compromised by the universal character. Using better-adapted dispersants can lead to significant amounts of TiO2 savings.
The optical effectiveness of TiO2 depends on the design and quality of the grade considered. Selection tables or technical data sheets help choose the proper TiO2 grade. The claimed characteristics and features correspond to the powdered pigment as it is designed and produced. The same should be recovered once the pigment is dispersed in water.
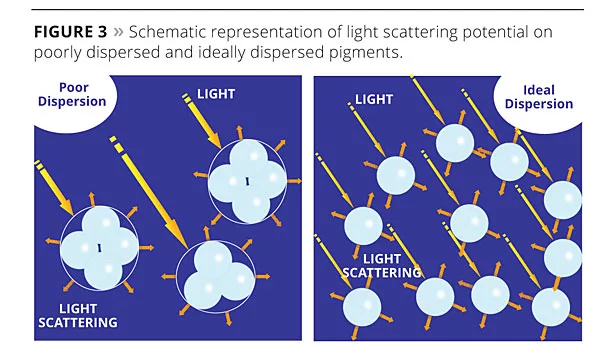
Optical effectiveness depends on light scattering, which is enhanced with the use of very fine mineral particles (less than 1 micron). Light scattering efficiency comes from reflected and refracted light onto small particles, and diminishes when particles become bigger. This is the case as well for agglomerated particles, which can be assimilated to larger-sized particles (Figure 3). Agglomerated particles remain when the powdered pigment has not been deflocculated properly at the mill base stage, or form when pigment particles begin to flocculate once dispersed in the water phase. In either case, successful deflocculation is based not only on the stirring efficiency and time but also on the proper selection of the dispersing additives. When chosen dispersants are not fully adapted to the specific paint formulation, TiO2 opacity is compromised.
Using the Co-Dispersant Approach
The co-dispersant approach combines the use of an acrylic-type dispersant (homopolymeric or copolymeric) and a specific additive technology developed by Coatex called Bumper Technology™. Bumper Technology™ is a new proprietary dispersing technology platform developed to help formulators reduce TiO2 levels in coatings. This patented additive technology optimizes dispersion and prevents particle flocculation, while using less pigment, and maintaining or improving critical optical properties of the paint. Bumper Technology™ is compatible across a wide PVC and binder range.
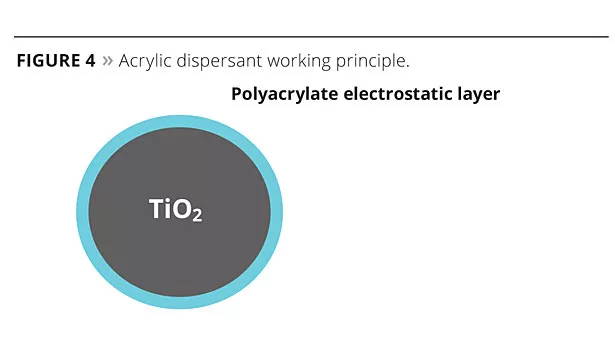
Acrylic-type dispersants enable the reinforced electrostatic repulsion potential between the particles and, therefore, help deflocculate and stabilize TiO2 particles (Figure 4).
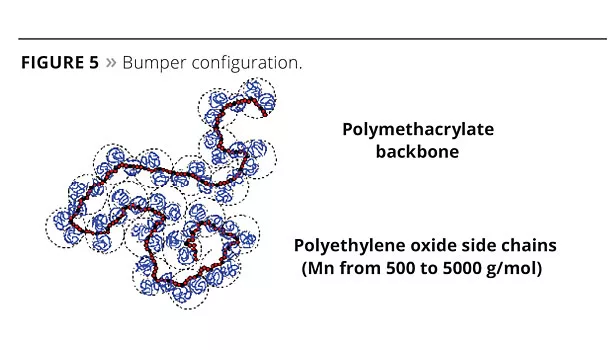
Bumper Technology™ (Bumper) shows a low ionic character thanks to a reduced acrylic backbone on which long alkoxylated side chains are grafted (Figure 5). The acrylic backbone ensures the water solubility of the additive. Its accessibility is governed mainly by the spreading rate of the alkoxylated chain (PEG, i.e., polyethylene oxide glycol chains), making it easily accessible or not to the cations.
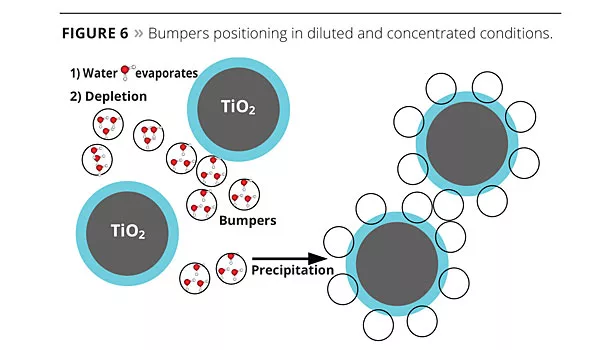
When the water begins to evaporate and the coating starts to dry (high concentration conditions), PEG chains force the polymer to precipitate on the mineral surface as the osmotic pressure becomes too high (Figure 6). PEG chain length can tune the precipitation rate, and the molecular weight of the Bumper the spacing distance.
In Figure 7, the needed distances for good TiO2 spacing at the end of the drying process are reported. The targeted dimension for the Bumpers is in the 20-40 nm range.
The first commercially available Bumper is Coadis™ BR 85, in which the particle size is situated at the lower limit of the effective spacing range. Therefore, Coadis™ BR 85 can be considered both as a dispersant and as a Bumper for TiO2.
Bumper Technology™ is a flexible technology platform that enables the ability to adapt the Bumper additive’s design for specific spacing of TiO2 particles in various conditions. In particular, starting from the design of Coadis™ BR 85, the molecular weight of the Bumper can be increased in order to cover the full range of spacing distances, or its structure can be modified in order to fine tune possible interaction with TiO2 particles or other ingredients of the paint (e.g., imparting to the Bumper a more or less hydrophilic character).
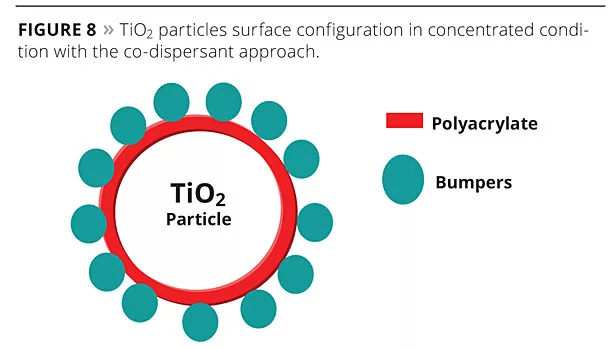
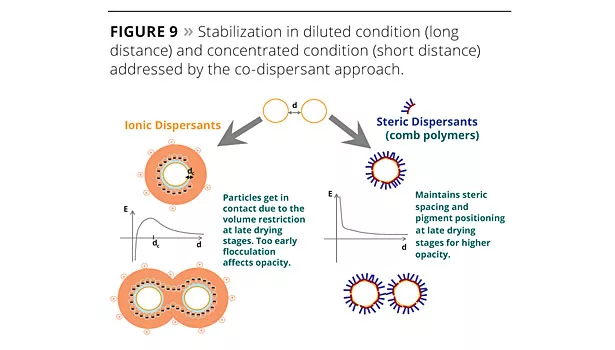
The co-dispersant approach (Figures 8 and 9) is focused on the pigment stabilization and spacing only, and therefore is less formulation-dependant than other approaches such as the use of plastic pigments or binders interacting with TiO2.
The following case studies highlight the benefits that can be achieved with the co-dispersant approach and Bumper Technology™.
Formulation 1 - Interior Eggshell, PVC: 40%
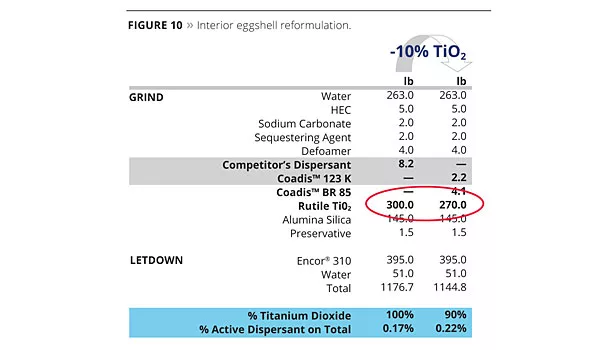
Opacity and tint strength of an interior eggshell paint based on a vinyl acrylic binder is maintained, even with a TiO2 reduction of 10% and replacement of a standard dispersant by a co-dispersant system. Using the co-dispersant approach, the total amount of dispersant is slightly higher, but with no significant impact on the cost saving corresponding to a reduction of 10% of TiO2. There is no added mineral to compensate the missing TiO2, therefore there is a slightly lower pigment volume concentration (PVC) after reformulation (Figure 10).
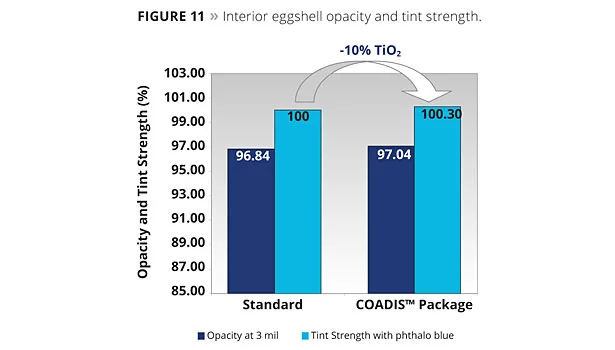
As can be seen from Figure 11, opacity and tint strength are maintained despite the 10% TiO2 reduction and the lower PVC. We see better spacing of TiO2 during the latter stages of the drying process of the coating, thanks to the co-dispersant approach and the Bumper Technology™ introduction.
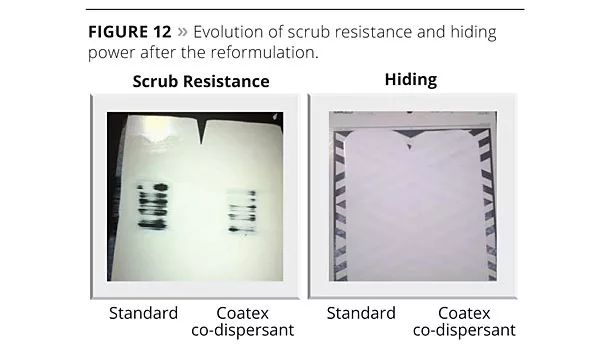
Figure 12 highlights no difference in terms of hiding power and even increased scrub resistance when using the co-dispersant system.
Formulation 2 - Interior Eggshell, PVC: 40%
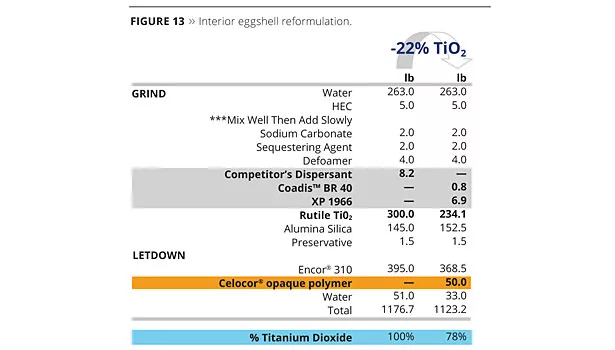
Formulation 2 is very similar to Formulation 1, however the reformulation work is deeper due to the extreme high target in terms of TiO2 savings – 22%. Therefore, the Coatex codispersant approach was combined with a plastic pigment (opaque polymer) replacement strategy, as shown in Figure 13.
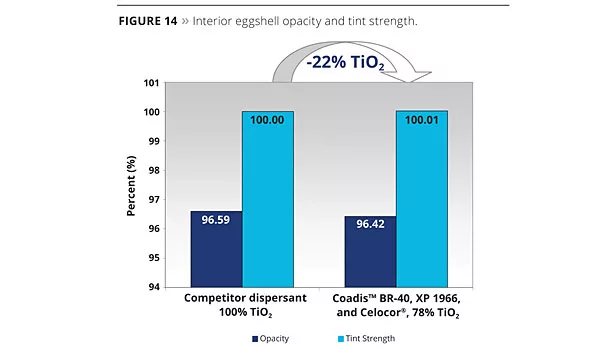
The experimental Bumper XP 1966 was selected for the synergistic effect it could build with both the copolymeric acrylic dispersant (Coadis™ BR 40) and with the plastic pigment (Celocor®). It’s specifically tuned molecular weight contributes to improve the interfacial interaction between the TiO2 particles and the plastic pigment. As a result, an impressive 22% reduction in TiO2 could be achieved while not affecting the opacity and tint strength (Figure 14). Figure 15 is a micrograph of the Celocor® and Bumper Technology™.
Conclusion
The co-dispersant approach supported by Bumper Technology™ opens new perspectives for formulators looking for innovative and effective TiO2 savings. Unlike existing strategies, it is focused on the valorization of TiO2 particles by allowing them to develop their full potential in terms of optical opacity, both in dilute conditions (paint storage) and in the highest concentrated conditions (paint during drying).
The co-dispersant approach implementation can help save up to 22% of TiO2 or more if it is associated with other compatible strategies such as plastic pigment incorporation.
The authors would like to thank Dr. Olivier Guerret, previously Research & Development Director for Coatex Group, for his personal contributions to the development of Bumper Technology™.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!







