The Role of Molecular Defoaming Actives
in Low-VOC Paint Defoamer Design

Webster’s dictionary defines foam as “A light, frothy mass of fine bubbles formed in or on a surface of a liquid.” Foam is further described as “a stabilized froth produced chemically or mechanically.” These descriptions hold true when discussing foam entrapped in coatings. When discussing coatings, foam is further defined as the dispersion of a relatively large volume of gas (air) in a small volume of liquid. In whichever way that foam is defined or described, it is a serious problem in both the wet and dry state of the coating when it is not removed. Foam that remains in the dried coating film leads to detraction in the coating properties, which in turn can result in premature coating failure and catastrophic effects. This article will show how a class of molecular defoamers proves to be a solution to removing foam from coatings in an effective, efficient and persistent manner. We will first review the causes of foam, discuss the typical components in a defoamer, explain how defoamers work in the coating system and then show examples of the effectiveness of defoamers that contain a molecular-containing active compared to control defoamers that do not contain the molecular active in their composition.
There is much literature discussing the differences between antifoams, defoamers and deaerators. Acceptable definitions are that an antifoam is an ingredient that prevents or retards the formation of foam, a defoamer acts on foam after it has been formed and reduces or eliminates it, and a deaerator aids in intensifying bubble coalescence to accelerate foam release. In reality, an additive made to reduce and eliminate foam works in multiple ways and it becomes difficult to classify the additive, therefore in this article, the term defoamer will be used to describe the additives discussed.
Foam Causes
Pure liquids do not hold foam. This is easily seen when you shake water. Bubbles form and they quickly rise to the surface of the water and break. There is no tendency for the water to hold the air; it quickly rises and leaves the liquid. Coatings, on the other hand, are not pure liquids. So what is the cause for coatings to hold onto the air and trap bubbles within the coating liquid or dried coating film?
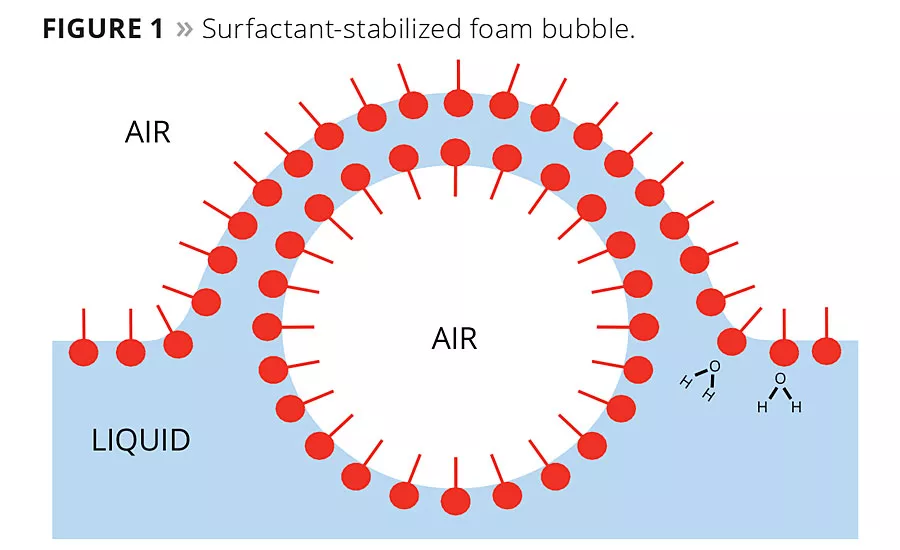
Water-based coatings are not pure liquids; they are a colloidal dispersion of particles (pigment, resin) in a liquid carrier (water). One of the key factors in stabilizing foam in these coatings is the same factor that keeps all of these dissimilar ingredients together and not phase separate or settle: surfactants. Surfactants are ingredients used to keep latexes from phase separating, to wet pigment surfaces to improve dispersion and stop settling, and to improve the overall compatibility of ingredients in water-based coating formulas. The negative aspect of these surfactants is that they are very effective at stabilizing air in the coating mix as well and make it very difficult for the air to agglomerate, rise to the surface and escape. This is due to the very nature of surfactants. Surfactants are materials that contain both hydrophobic and hydrophilic character. It is by this that they are effective at performing their function and also why they are effective at stabilizing foam. The hydrophobic portion of the surfactant surrounds the air bubble and stabilizes it in the coating liquid, thus preventing the bubbles from agglomerating and rising to the surface to escape (Figure 1).
Defoamer Composition
There are three basic parts to a defoamer formulation:
- The primary liquid;
- Emulsifiers and wetting agents;
- Tertiary or active components.
The primary liquid, or carrier, can serve as a defoamer on its own. In the defoamer formulation, its primary function is to carry the hydrophobic ingredients into the lamella interface where they can be effective. Examples of primary liquids are mineral oil and silicone oil. Emulsifiers or wetting agents are used to aid the dispersion of the defoamer into the liquids to be defoamed. They control the degree of difficulty to disperse the defoamer and also have an impact on the efficacy, persistence and compatibility of the defoamer. The tertiary components are items added to increase the performance of the defoamer, improve the defoamer stability or compatibility. Items such as hydrophobic particles, fatty acids, coupling agents and proprietary components, such as defoaming molecules, fit into this category.
How Defoamers Work
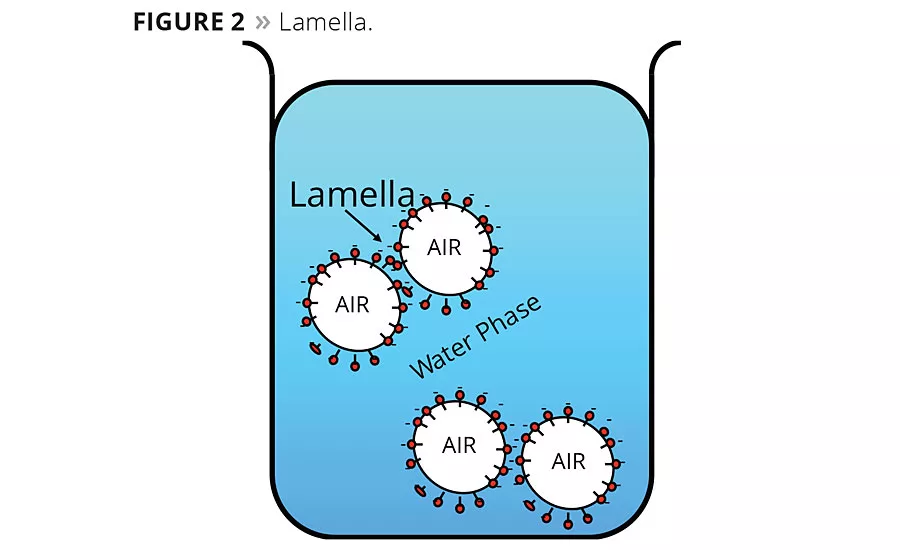
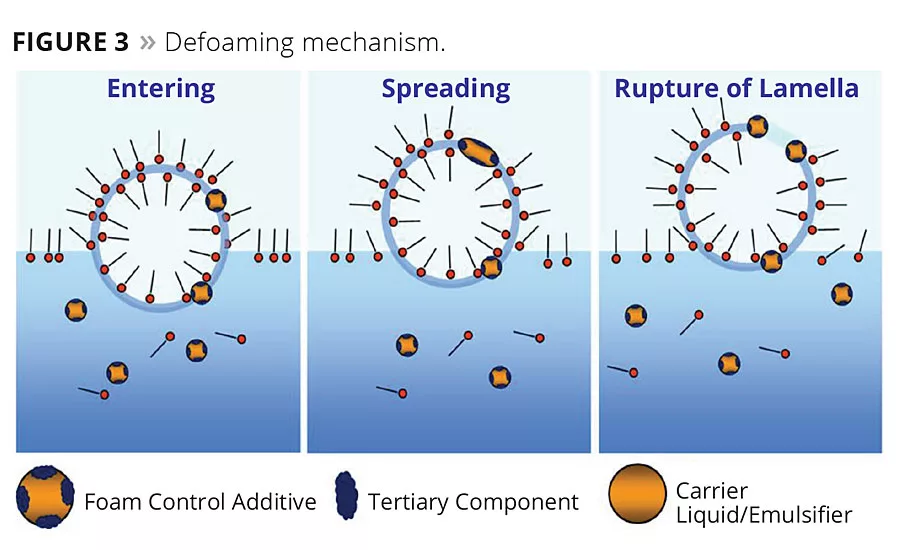
It is the defoamer’s job to overcome the stabilizing effect of the surfactants and remove the air from the system. To do this, defoamers are formulated to be incompatible with the surfactant layer interface between the bubble wall and the surface of the liquid or a neighboring bubble wall. This area is commonly referred to as the lamella (Figure 2). Defoamers need to be low-surface-tension liquids that have controlled insolubility or incompatibility, positive entering coefficient to enter the lamella and positive spreading coefficient to spread across the lamella. Defoamers also need to be dispersed as hydrophobic droplets in the formula. Once the defoamer droplet enters and spreads across the lamella area, it disrupts the surfactant stabilization, causing the water to drain from the lamella, resulting in thinning of the bubble wall and eventual rupturing of the bubble (Figure 3). Thus, in addition to surface tension of the defoamer, a key characteristic for effective defoamer performance is that the defoamer is capable of being dispersed in the liquid being defoamed to the correct and effective droplet size.
There are five basic methods of defoaming activity:
- Hydrophobic solids;
- In-situ formation of hydrophobic solids;
- Competition for surfactant;
- Low-surface-tension liquids;
- Antagonistic surfactants/polymers.
Molecular defoamers fall into the last category, antagonistic surfactants/polymers. This technology is effective by the defoaming molecule interacting with other surfactants in the coating to form a rigid film rather than an elastic film in the lamella wall. This is achieved by a repulsive interaction occurring between the surfactant chains. Thus, the lamella area surrounding the bubble does not stretch and is more prone to burst open and release the air. As a result, the difficulty in finding effective and efficient defoamers is to balance this incompatibility needed in the coating system, with the defoamer’s ability to overcome the stabilizing effect of surfactants, without causing any defects in the coating system, such as craters, crawling, colorant separation, etc.
Low-VOC Coating Defoamer Needs
With the rapidly evolving regulations regarding VOC emissions and consumer demand for more environmentally friendly products, coatings have changed dramatically in the past decade, to the point today, where architectural coatings are being formulated to contain near-zero-VOC content. Latexes used are softer polymers that do not need coalescent to achieve film formation and develop hardness by post-application crosslinking. Solvents for open time extension and maintaining wet edge during application are being eliminated. All coating ingredients are expected to be at or near zero VOC, leaving water as the only liquid volatile component in the formula. The use of surfactants becomes more critical at stabilizing these formulas, and the use of environmentally friendly surfactants has changed the landscape on foam generation and stabilization. In architectural coatings, one-coat coverage is now expected, and one of the methods used to achieve this is to formulate coatings at higher viscosities than in the past, in both the low and high shear ranges. These factors have all had an effect on the defoaming needs of modern coatings. Solvents may no longer be present to act as temporary defoaming agents in the formula, especially during the manufacturing process. Higher viscosities mean that the rate of bubble rise out of the liquid is slower and more difficult. Increased use of surfactants means that foam is more stabilized throughout the coating. Defoamers must now be formulated to counteract all of these effects.
Molecular-Based Defoamers
The use of molecular defoaming agents as antagonistic surfactants or polymers is an answer to today’s defoamer needs. Molecular defoamers are able to work on a much smaller scale compared to typical defoaming actives, which are usually particulate in nature. This smaller scale of the molecular defoamers dramatically improves their efficiency in the defoaming process. Molecular defoamers are used in combination with multiple classes of defoamer technology – mineral oil based, silicone based and water emulsion form. The effectiveness of these modern defoamers is superior to the defoamers that do not contain a molecular defoaming component. The following data is based on the use of a silicone-free, hydrocarbon-based molecular defoaming hyper-branched polymer as the active molecular component.
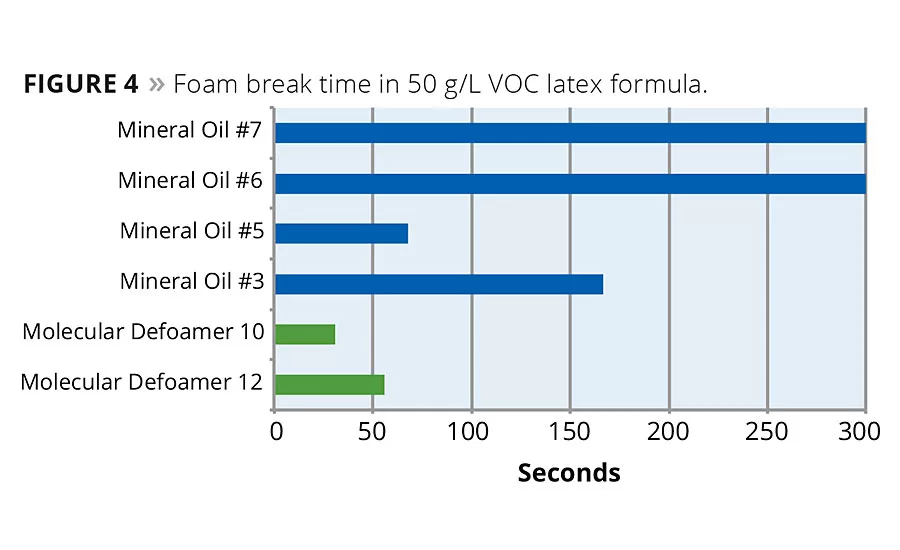
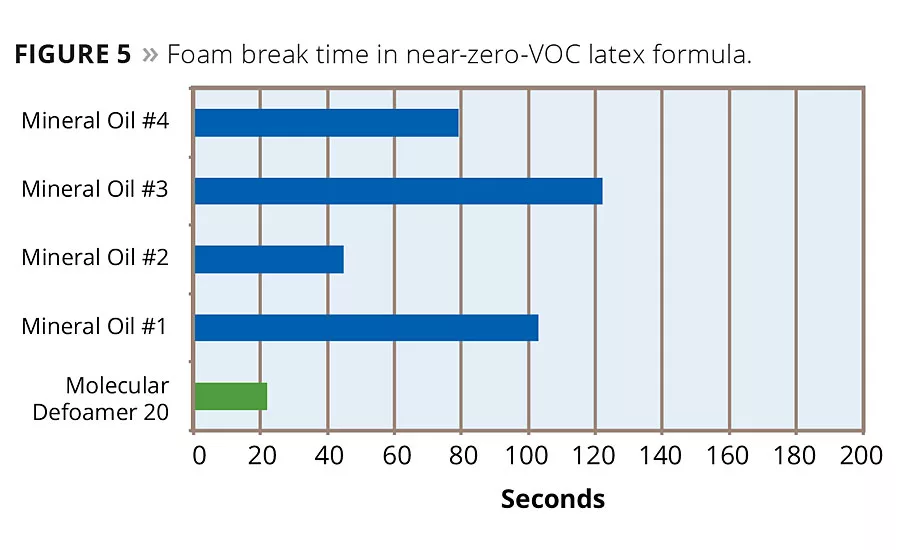
Adding a molecular defoaming component to mineral oil technology improves the bubble break time in low-VOC latex formulas. Results in a 50 g/L VOC flat acrylic latex formula show this effect. Comparing defoaming effectiveness to commercially available mineral oil products, the defoamers that contain molecular actives outperformed the standard mineral oil defoamers. The best mineral oil defoamer had a bubble break time of 68 seconds, while the two molecular actives containing defoamers had bubble break times of 56 and 31 seconds respectively (Figure 4). Examining the applied dried film, the molecular defoamers were also better at eliminating entrapped microfoam in the coating. In a second formula, a near-zero-VOC flat acrylic latex, results are similar. The best commercially available mineral oil defoamer broke the foam in this system in 44 seconds, while the defoamer containing the molecular component broke the foam in 25 seconds (Figure 5). Again the dried film was left with less microfoam entrapped in it.
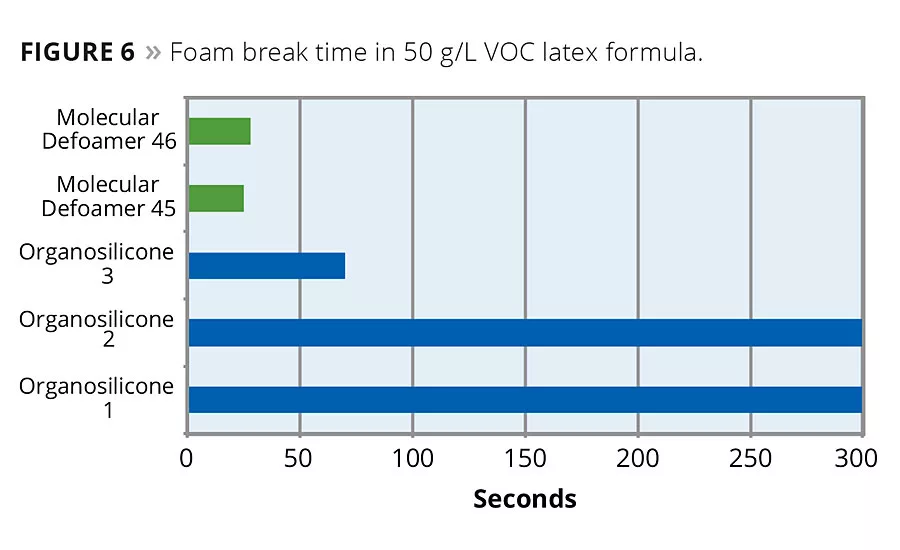
Evaluating defoamers based on silicone chemistry, we also see an improvement in performance when a molecular component becomes part of the defoamer formula. In a 50 g/L VOC acrylic semigloss latex formula, two defoamers containing a molecular component outperformed three commercial silicone-based defoamers. Two of the commercial products did not break the foam bubbles generated under application conditions. The third commercial product took 70 seconds to break the foam. The two silicone defoamers that contain a molecular component broke the foam in 25 and 28 seconds respectively (Figure 6).
Evaluating the products in a near-zero-VOC acrylic semigloss formula, we see even more dramatic differences in the defoamer that contains molecular actives. The commercial silicone products, used at a loading level of 0.5% by weight on total weight of coating, could not break the application foam, leaving visible bubbles in the dried coating. The molecular actives containing silicone defoamer remained effective in this system, breaking the foam in 68 seconds, and it was used at a loading level of only 0.3% by weight on total weight of the coating (Figure 7). The defoamer containing molecular actives was thus more effective at breaking application foam even at a 40% reduction in loading level.
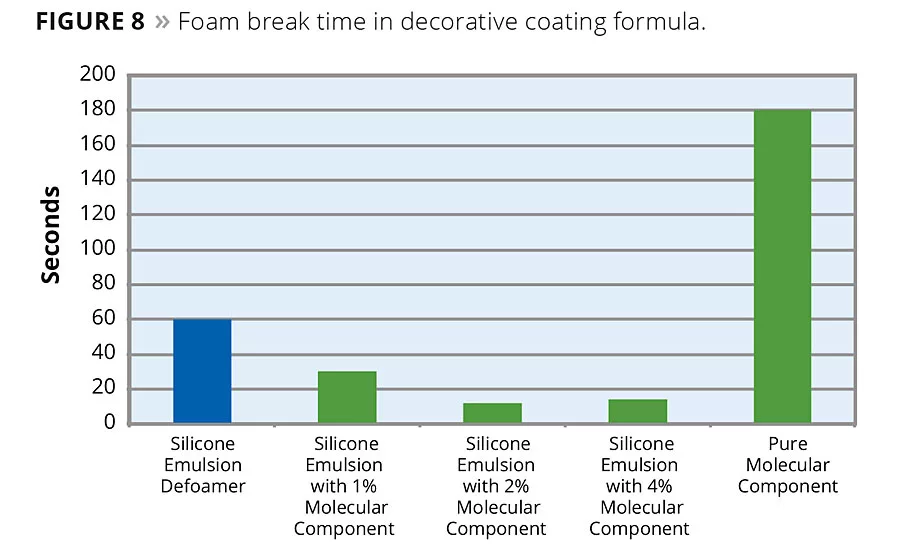
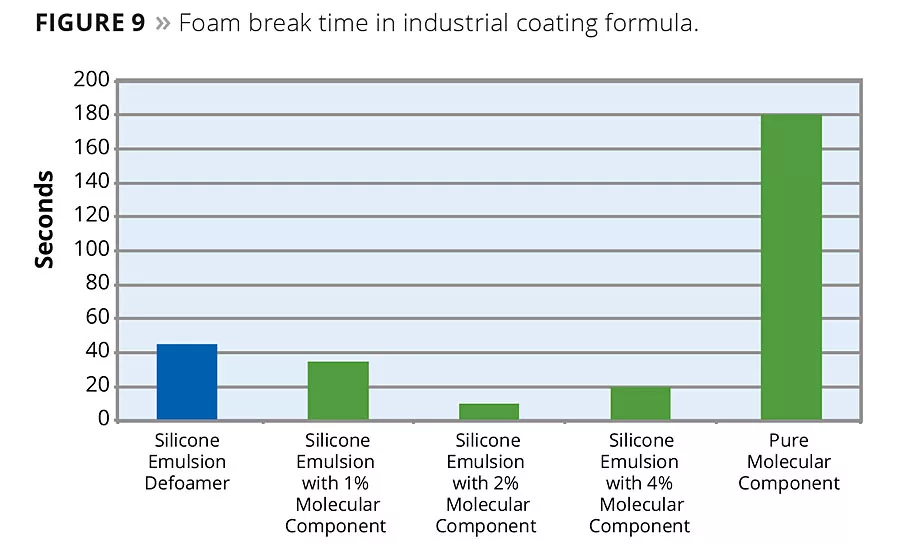
Silicone emulsion defoamers are another area where the molecular defoaming component has shown a significant impact on defoamer performance. In an acrylic latex formula, one for architectural or decorative coatings and another for industrial coatings, the molecular defoaming component shows a significant improvement in application foam reduction. The following series shows the effect of addition of various levels of molecular defoaming component to a defoamer formulation. As the results show, the molecular component gives an immediate boost to defoaming activity, yielding maximum effect at 2-4% molecular component based on total defoamer formula weight. In the decorative formulation, bubble break time was reduced from 60 seconds to a low of 12 seconds (Figure 8). In the industrial formulation, bubble break time was reduced from 45 seconds to a low of 10 seconds (Figure 9).
As is shown, the molecular defoaming component used alone had very poor defoaming performance. This shows that the molecular actives need to be properly formulated in a defoamer composition in order to achieve positive and efficient defoaming effects.
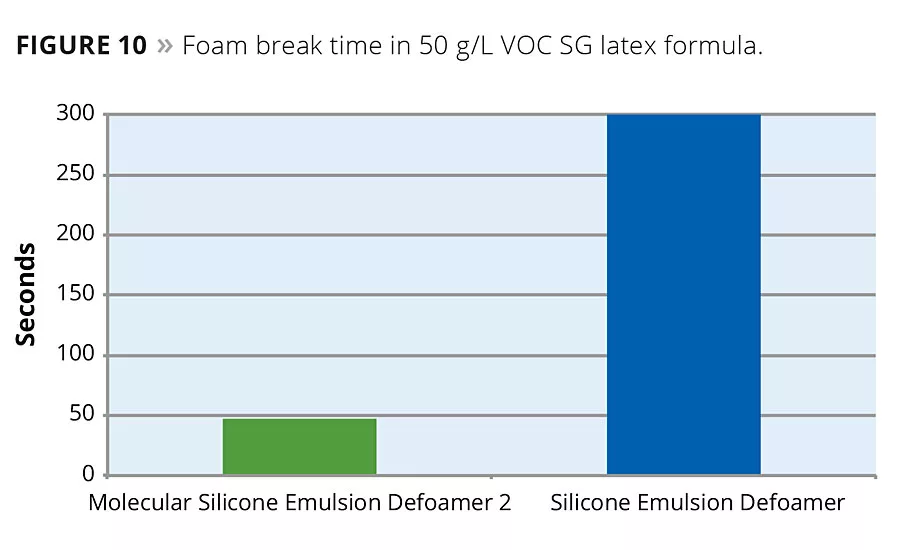
The silicone emulsion defoamer was also evaluated in two architectural formulas, both 50 g/L VOC. The first is an acrylic semigloss, and the defoamers were loaded in the system at 0.75% by weight as supplied on total coating formula weight. It is important to note that the silicone emulsion defoamers in the test are supplied at 20% total actives, so adding 0.75% as supplied is only adding 0.15% actives on total formula weight. As can be seen, even at this low usage level, the silicone emulsion defoamer that contained the molecular component as part of its actives was effective at eliminating application foam in the coating, while the silicone emulsion defoamer without any molecular component did not eliminate the foam (Figure 10).
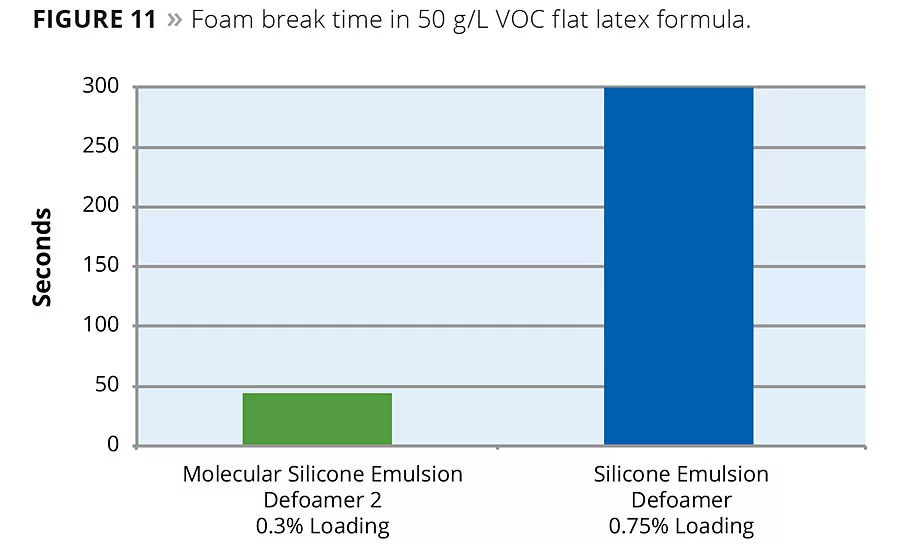
The same defoamers were evaluated in a second formula, a 50 g/L VOC flat acrylic system. In this system, the defoamer containing a molecular defoaming component was able to eliminate application foam at a use level of 0.3% as supplied, or 0.06% actives. The silicone emulsion defoamer without the molecular defoaming component did not eliminate application foam, even at a use level of 0.75% as supplied (Figure 11).
Conclusion
Defoamers that contain a molecular defoaming component as part of their composition show an ability to defoam more efficiently than their non-molecular defoaming-containing counterparts. This allows for lower defoamer usage in coatings and fills a critical need as the VOC of coatings is reduced. The higher degree of difficulty in defoaming low-VOC coatings is solved by the use of molecular defoamers, especially as the VOC of coating formulas approaches zero.
References
1. Orr., E.W. Performance Enhancement in Coatings. Cincinnati: Hanser/Gardner Publications, Inc. (1998).
2. Breindel, K. Sugar Coating Molecular-Based Defoamer Eliminates Foam and Enhances Coating Properties. Modern Paint and Coatings, (May 2000). 18-22.
3. Martens, C. R. Waterborne Coatings. New York: Van Nostrand Reinhold Company, (1981).
4. Webster’s New Collegiate Dictionary. (1976). G. C. Merriam Co.
Special thanks go to Daniel Herzog, Senior Lab Chemist, Mike Wiggins, Senior R&D Chemist, and Petra Loddenkemper, Laboratory Technician, for their work on developing the defoamers, running the evaluations presented and contributions to this article.
This article originally appeared in the December 2014 issue of APCJ.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!






