Wetting and Dispersing Additives
Past and Future Challenges


Figure 1

Figure 2

Figure 3
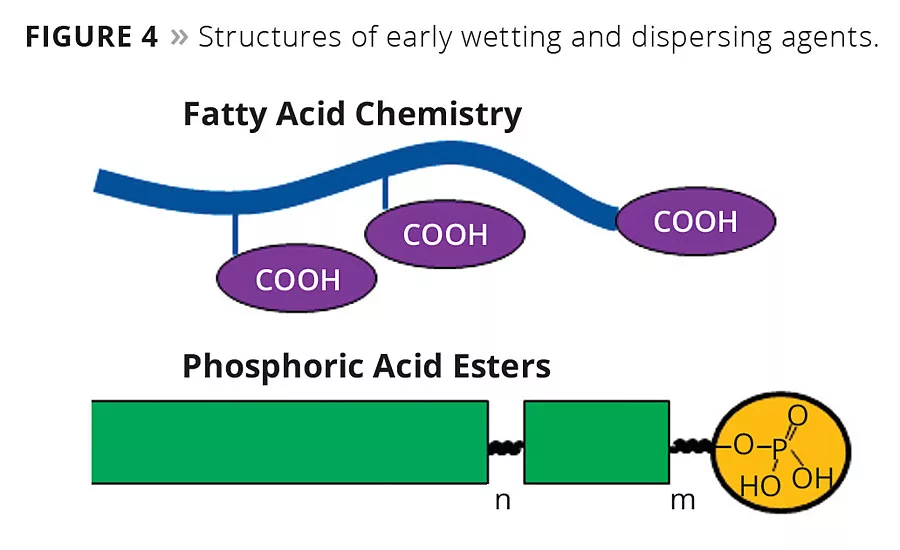
Figure 4
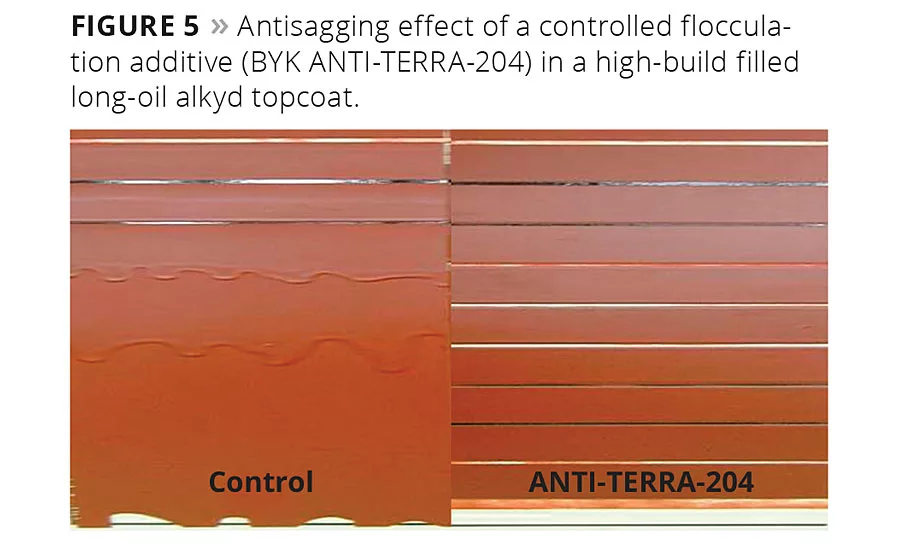
Figure 5
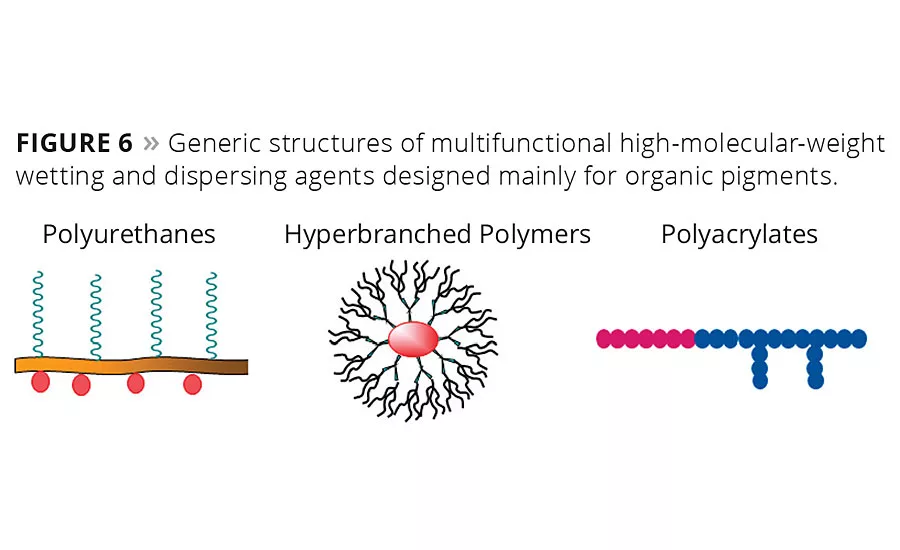
Figure 6
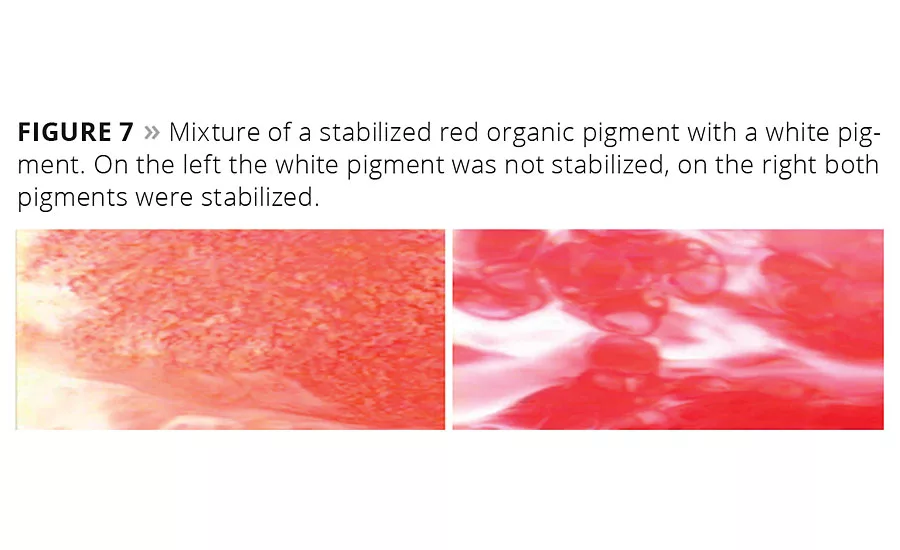
Figure 7
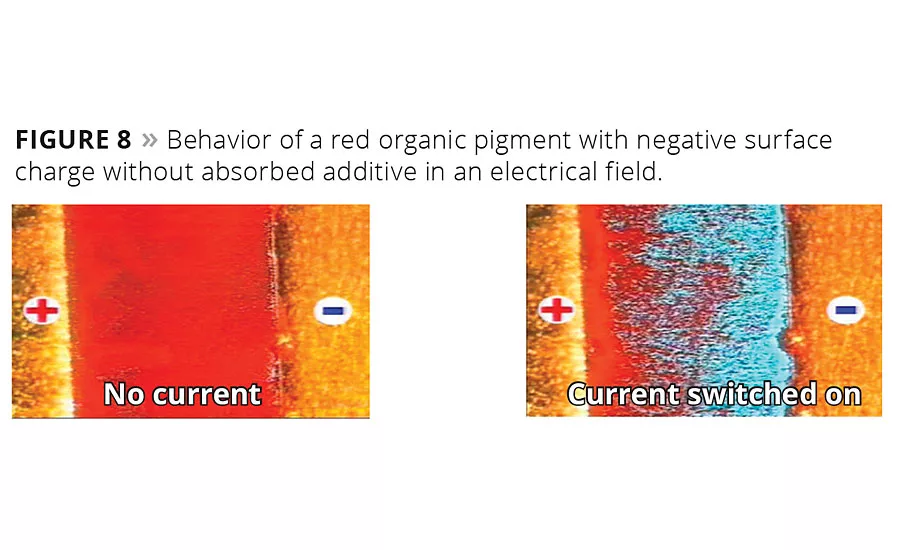
Figure 8
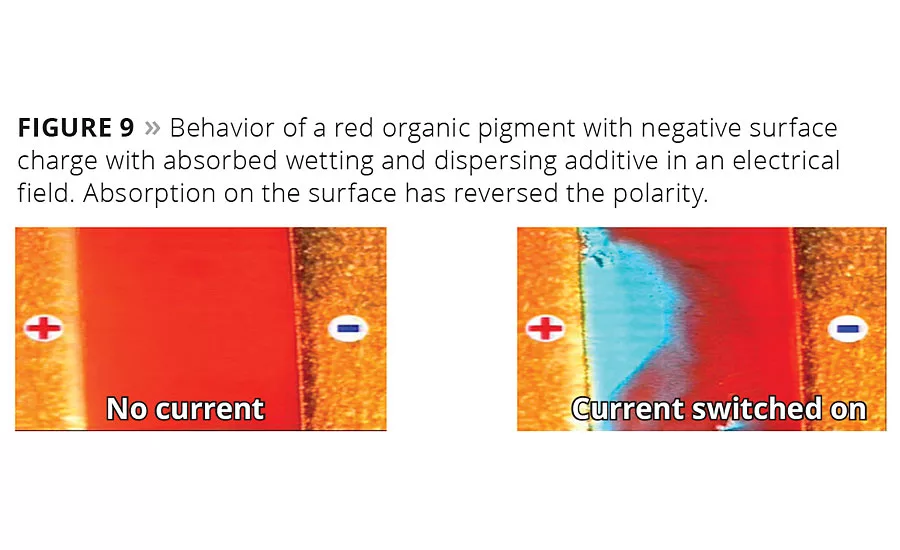
Figure 9
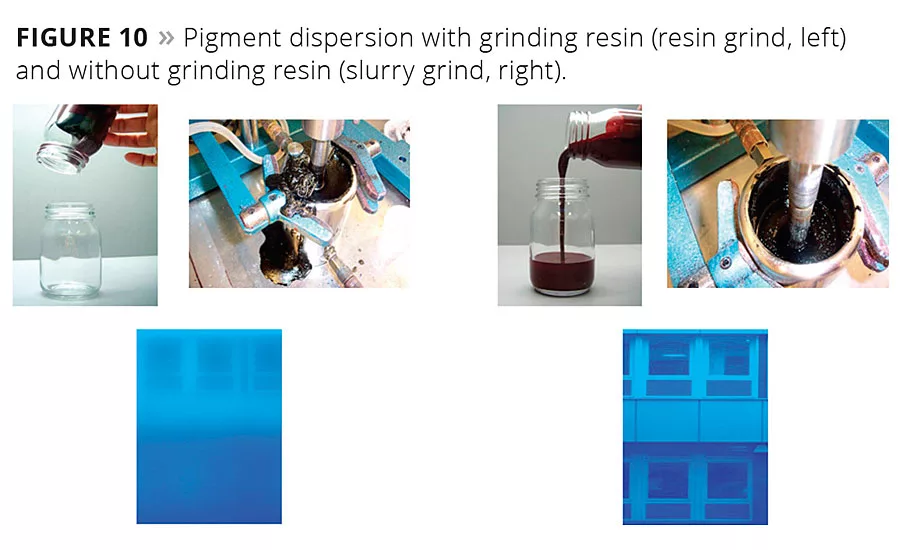
Figure 10
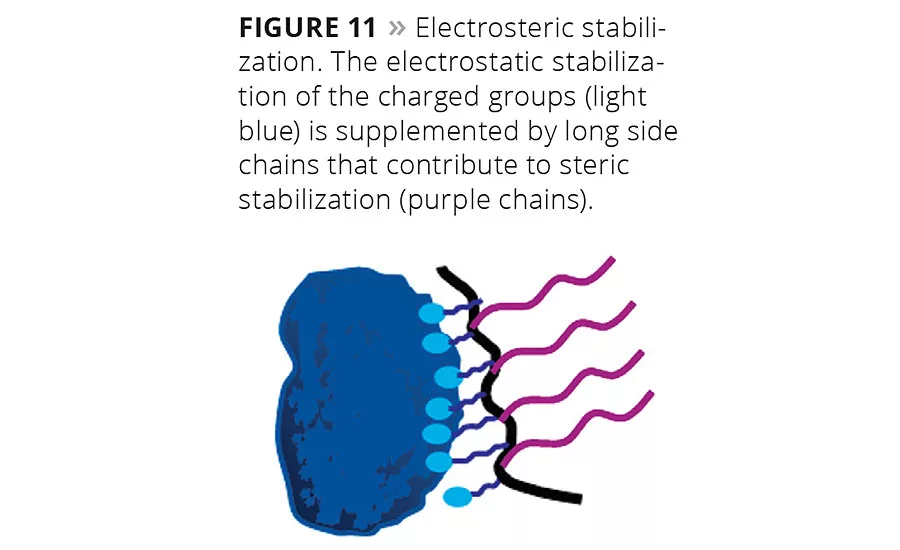
Figure 11

Figure 12
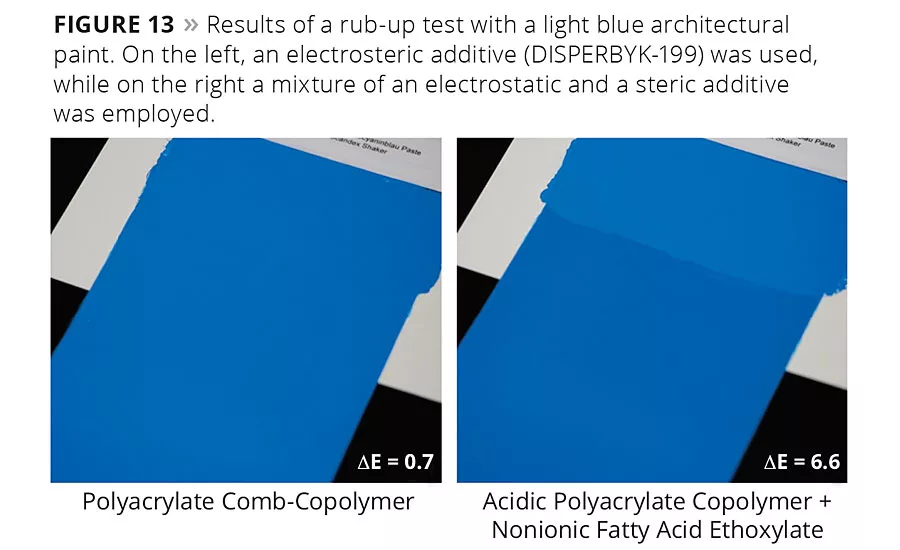
Figure 13
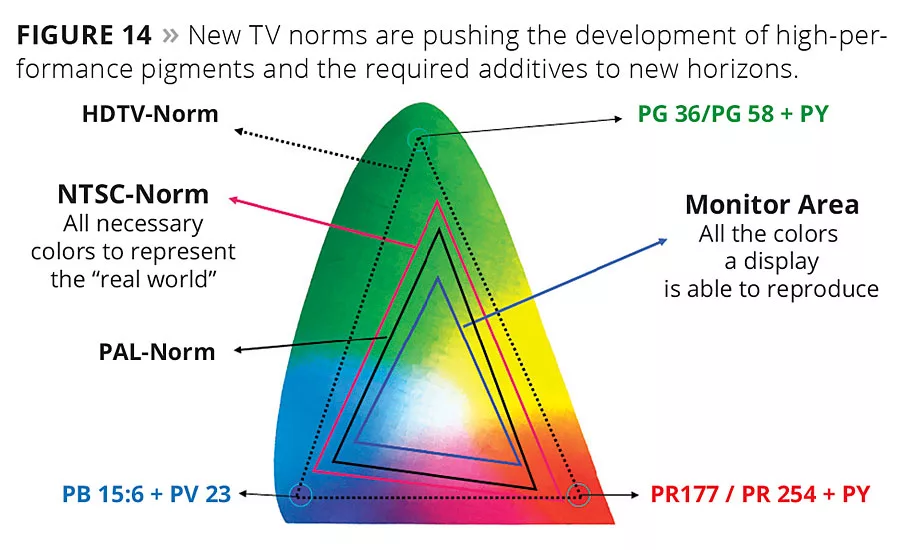
Figure 14

Figure 15
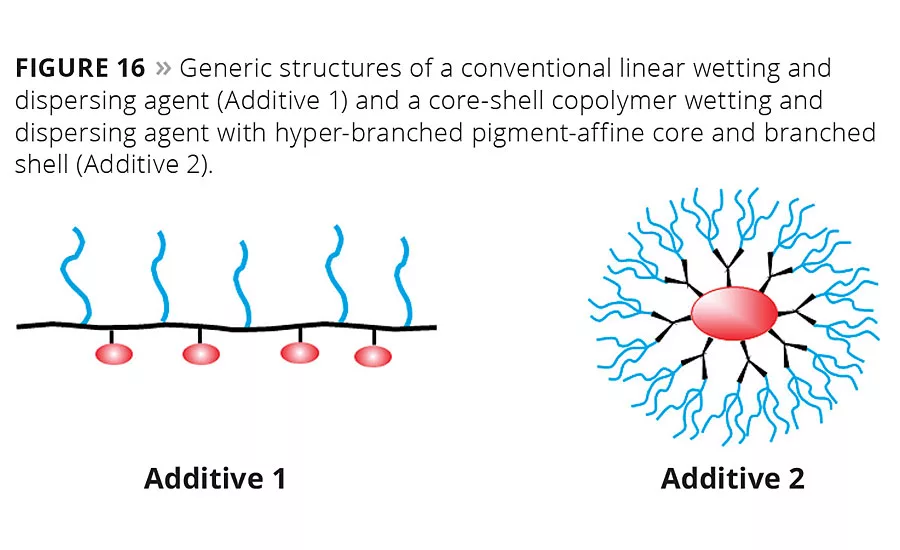
Figure 16
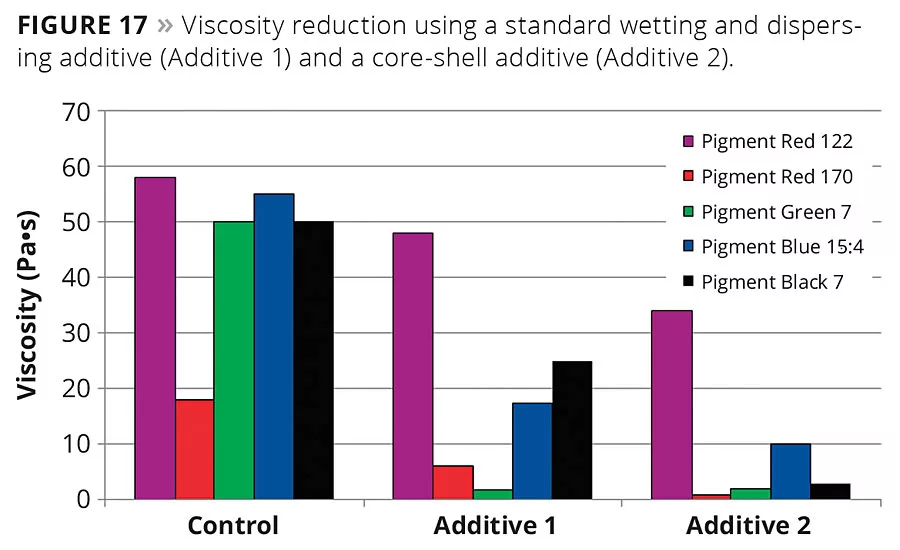
Figure 17
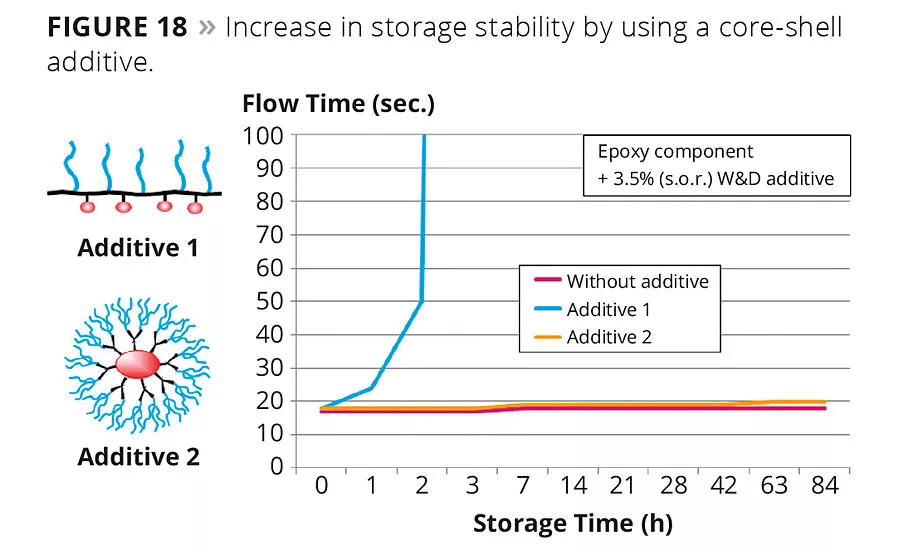
Figure 18
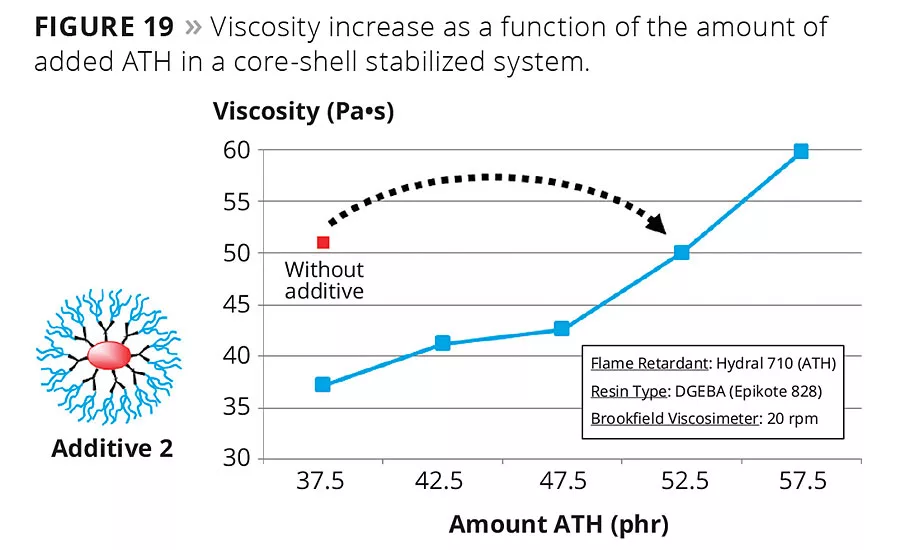
Figure 19
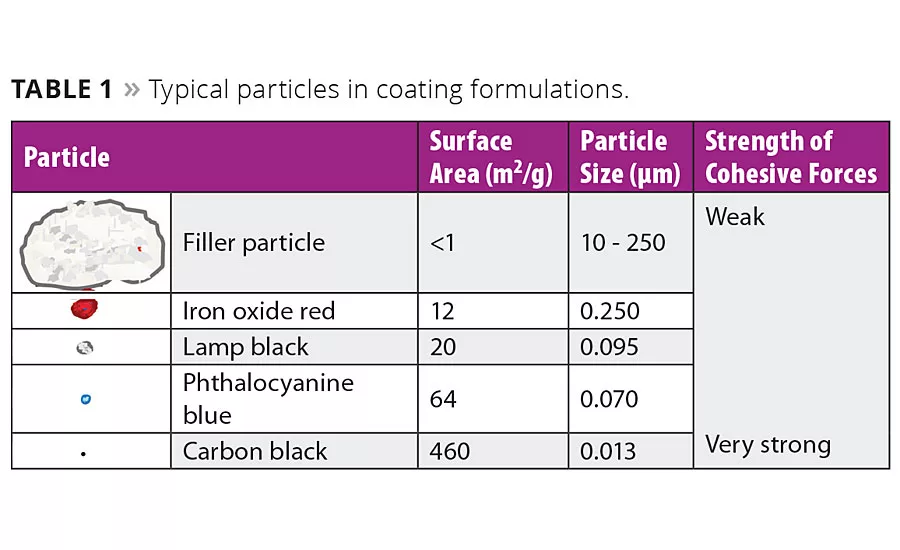
Table 1
Without wetting and dispersing additives, pigments and fillers in paints could not be properly dispersed, and once dispersed, would not remain evenly suspended in the paint. The demands on pigment dispersion agents in modern binders are ever increasing, and requirements regarding color reproducibility are becoming more stringent. A wide range of chemically distinct pigment wetting agents, dispersants, and wetting and dispersing additives have been developed over time.1 Current product development focuses on the development of environmentally friendly, VOC-free and higher solids products. New polymerization techniques (e.g. group transfer polymerization) provide access to tailor-made, highly effective block copolymer additives with superior performance. This article presents a short overview of the wetting and dispersing process, the development of different additives over time and more recent developments.
The Wetting and Dispersing Process
Pigments for coatings are typically supplied as powdered materials wherein primary particles form larger agglomerates. These cohesive forces within a pigment agglomerate increase with decreasing particle size. This effect is most pronounced for organic pigments such as carbon blacks. The wetting and dispersing agent has to enable wetting of these particle surfaces, stabilize them and warrant a satisfactory performance of the coating. Table 1 shows the dimensions of typical particles that are dispersed and stabilized in coating formulations.
To achieve good wetting of pigment agglomerates and primary particles, the surface tension of the resin (or liquid carrier) must be lower than the surface energy of the pigment. Inorganic pigments and extenders have a relatively high surface energy of 50 mN/m to 90 mN/m. They are therefore much easier to wet than organic pigments or carbon blacks, which have a lower polarity (surface energy of 25-35 mN/m). Strongly agglomerated small pigment particles are always more difficult to wet than less agglomerated or larger primary particle size pigments. As the binder penetrates into the pigment agglomerates and wets the surface of the primary particles, the wetting stage is continuously transformed into the pigment dispersing stage. The pigment dispersion stage always requires the input of energy to break down the agglomerates. Small pigment particles that are strongly agglomerated always require more energy during the dispersion stage.
Dissolvers can be utilized to grind extenders or inorganic pigments with a larger particle size. For organic pigments, carbon black and transparent iron oxides, three-roll mills, sand mills, pearl mills, etc. are required. Deflocculation is achieved when the primary particles are individually distributed within the paint or binder.2 Completely deflocculated pigments provide many technical benefits in coatings and inks, such as maximizing the opacity and color strength of opaque pigments and providing the best possible transparency and color strength of transparent pigments. They increase gloss, reduce haze, prevent color separation, flooding and floating, and create a more Newtonian rheology that leads to better leveling properties.
Primary particles brought into contact with each other re-associate within a few seconds to form flocculates due to London-van der Waals forces, hydrogen bonding and electrostatic attractive forces (pigment/pigment flocculation). In order to establish resistance to flocculation throughout the entire production process, storage and application, suitable additives must be incorporated. Figure 1 depicts the difference between a suitably stabilized pigment and a pigment that re-associated.
Two different stabilization mechanisms – controlled flocculation and deflocculation – are used in all types of coatings to prevent deflocculated systems from spontaneous flocculation.3 Figure 2 depicts the working mechanism of controlled flocculating and deflocculating additives.
Controlled flocculating additives with branched structures and carboxylic acid-based pigment-affine groups establish secondary network structures. These networks, which are based on hydrogen bridges between polar functional groups, prevent pigment settling and improve sag resistance. High-density inorganic fillers, in particular, need the combination of a wetting agent with a controlled flocculating additive to prevent hard settlement.4 Figure 3 lists typical types of wetting and dispersing agents, their chemistry, structure and the resulting properties.
Inorganic Pigments in Solventborne Systems
Initially, pigments of natural origin were used to color paints (clay, earth colors, minerals such as ultramarine, etc.). These inorganic pigments are similar in terms of their relatively coarse primary particle size and polar specific particle surfaces areas. The interaction of wetting agents and dispersants with these fillers and pigments is based on acid-base interactions. Low-molecular-weight molecules with only one acidic group were developed as wetting agents, and controlled flocculating additives based on fatty acid oligomers were introduced to the market.5
The top part of Figure 4 shows the generic structure of a controlled flocculating additive. The multiple carboxylic acid groups distributed along the polymer structure provide the pigment-affine functionalities and are responsible for forming the network needed for controlled flocculation. Typical wetting agents for inorganic pigments and fillers are linear copolymers based on polyethers, fatty alcohol ethoxylates or polyether-polyester block copolymers as depicted in the lower half of Figure 4 (green blocks). Usually, these wetting agents possess just one very polar pigment-affine group such as a phosphoric acid ester or its salt.
In primers or high-build topcoat systems, antisettling and sag resistance are very important, whereas high gloss of the coating is not critical. Figure 5 shows that in this case, controlled flocculating additives also impart excellent rheological properties. With increasing film thickness from top to bottom, the control on the left-hand side of the picture shows severe sagging. On the right-hand side, very good separation of the paint films is achieved due to the viscosity increase induced by the controlled flocculating additive.
Solventborne Applications – The Rise of Organic Pigments
The demand for brighter, more vibrant colors led to the development of synthetic organic pigments such as E anthraquinone yellow, phthalocyanine blue or perylene red during the second half of the 20th century. Synthetic organic pigments and mixtures thereof mean that virtually every color shade can be prepared.6
Typical organic pigments have a very large, relatively nonpolar specific particle surface compared with most inorganic pigments. Much weaker interactions with the particle surface require high-molecular-weight polymers with multiple pigment-affine groups or even pigment-affine polymer blocks (Figure 6). Typical pigment-affine groups are tertiary amino groups and their derivatives such as salts, quaternized ammonium structures and aromatic nitrogen-containing heterocycles.
Figure 7 visualizes the high absorption tendency of multifunctional high-molecular-weight additives on pigment surfaces. A red pigment concentrate is mixed with a white base. On the left-hand side only the red organic pigment dispersion is stabilized with a wetting and dispersing additive, and the inorganic pigment dispersion is not stabilized. The wetting and dispersing agent absorbed on the red pigment is strongly attracted by the relatively polar “free” surface that the inorganic pigment introduces into the mixture. The wetting agent leaves the relatively nonpolar organic red pigment and absorbs onto the inorganic surface. This results in flocculation of the red particles and poor coloristic properties. On the right-hand side of Figure 7 both the red and the white pigment are stabilized by suitable wetting and dispersing additives. Flocculation is avoided, and a very high-quality paint or tinting paste is obtained.
Additionally, the absorption of multifunctional high-molecular-weight additives, especially designed for stabilizing organic pigments, can be visualized in an electrical field. Figure 8 shows a dispersed organic red pigment without a strongly adhering wetting and dispersing additive. In the presence of an electric field the pigment particles migrate towards the positively charged electrode, indicating a negatively charged particle surface.
Figure 9 indicates that a suitable wetting and dispersing agent inverts the moving direction of the stabilized red pigment. The additive has strongly attached to the pigment surface and reversed the particle surface charge. It now moves towards the negatively charged electrode.
Waterborne Formulations
Increasing environmental concerns and regulatory restrictions led to the development of paint formulations with reduced amounts of organic solvents, water-dilutable formulations, waterborne formulations with cosolvents and completely waterborne formulations without cosolvents. These formulations exhibit a very polar character with different requirements for wetting and dispersing additives, especially in terms of compatibility and pigment stabilization.
Two different procedures are used to prepare a pigment concentrate for a waterborne formulation: resin grind and slurry grind. Figure 10 shows that even though the millbase formulations are similar, the grinding process and the coloristic properties of the resulting paints are usually different. Ideally, a widely applicable wetting and dispersing additive for water-dilutable or waterborne formulations should perform very well with both grinding methods.
Mechanisms of Dispersion Stabilization in Waterborne Systems
The first wetting and dispersing agents for waterborne applications employed electrostatic stabilization. Adsorbed additives carrying a positive or negative charge resulted in a charged pigment surface. The equally charged pigment particles create electrostatic repulsive forces that prevent flocculation, which is mainly applied in aqueous inorganic pigment or extender slurries. Polymeric dispersants provide a high degree of electrostatic pigment stabilization due to a high number of polar pigment-affine groups and a higher molecular weight. In addition, dispersants are able to chelate, especially multivalent cations to enhance the colloidal stability of the pigment dispersion.
Additives that incorporate long-chain structures inhibit the reflocculation of pigments based on steric stabilization. The longer and more coiled the chains, the better the stabilization. This stabilization mechanism is very important in systems with lower polarity.
Many modern water-based paint formulations require additives that combine electrostatic and steric stabilization, because the polarity of the system changes during drying. Figure 11 depicts a wetting and dispersing additive with a comb copolymer structure with polar pigment-affine groups and long binder-compatible dangling chains attached to the polymer backbone. This type of additive is widely used in many formulations and employs the electrosteric stabilization mechanism.
Figure 12 compares the performance of an additive with electrosteric stabilization (left), electrostatic (middle) and mixed electrostatic and steric using two different additives (right) in an aqueous white base tinted with a blue colorant. Only the polyacrylate comb-copolymer structure is capable of stabilizing both the inorganic white and the organic blue pigment in the aqueous formulation, while strong settling occurs with the other systems.
A rub-up test performed on a light blue architectural paint confirms this difference in performance: Figure 13 shows that for the same pigment mixture (organic blue and inorganic white) with two additives – one for electrostatic and one for steric stabilization – a pronounced color difference is observed. This is due to flooding of the blue colorant and settling of the white pigment. A polyacrylate comb-copolymer with electrosteric stabilization produces a significantly better result.
High-End Applications
The development of tailor-made pigments for very specific applications creates the need for specialty additives. One such example is flat-screen manufacturers, which employ semitransparent organic nanoscale pigments with very narrow particle size distribution to achieve high color strength, brilliance and brightness (Figure 14). This application not only requires the stabilization of very small tailor-made particles, but the complex manufacturing procedures require inks with very low, consistent viscosities of around 10 mPa*s. The organic matrix and wetting and dispersing additives have to resist harsh curing conditions with cycles above 200 °C without negatively impacting the coloristic performance of the pixel array.
This type of high-end application requires well-defined AB-block copolymer additives with a polydispersity as narrow as 1.1–1.3 and perfect block separation. To achieve such ambitious properties, controlled polymerization techniques have to be employed.7
Another example of a high-end application comes from the automotive industry. A brilliant, deep black color with a bluish undertone known as “jet black” is desired for high-quality car coatings. Stabilizing black pigments in the form of nanoscale carbon black particles with a very high specific surface area and a relatively nonpolar surface is difficult. High-solid solventborne basecoats with extenders and modifiers such as cellulose acetate butyrate and challenging grinding conditions make pigment stabilization even harder. Figure 15 emphasizes that only very specifically designed, tailor-made wetting and dispersing additives (right-hand side) can provide the required properties.
Solvent-Free, Low-VOC Additives
Printing inks for food packaging, decorative paints and many wood coatings are strongly affected by health and environmental concerns that lead to ever-increasing regulatory demands. One such example is universal tinters (pigment concentrates) for architectural paints with alkyl phenol ethoxylate-based wetting agents. These became a target of environmental concern and were replaced in most formulations by polymeric wetting and dispersing additives. The major current trend is towards VOC-free universal tinters (free of solvents, glycols or low-molecular-weight polyglycols that also contain volatile organic ingredients).
There is an extremely wide polarity gap between modern VOC-free aqueous emulsion paints that strongly shift their polarity during the curing process, and classic solventborne architectural paints. To provide a wetting and dispersing agent that can be used in all these formulations, sophisticated polymerization technology and unique building blocks are required. Side chains with different polarity need to be combined in one molecule to provide good stabilization from the highly polar aqueous starting point to the nonpolar phase after inversion. One type of structure with which many of these requirements can be fulfilled is a so-called core-shell copolymer (Figure 16).
The molecular structure of the additive has to be designed to obtain fast wetting of the pigment agglomerates, strong viscosity reduction during grinding and outstanding stabilization of the primary particles in the pigment concentrate, slurry or paste. The core-shell copoly-mers consist of a hyper-branched pigment-affine core and branched shell with a spherical shape. Their spherical shape leads to additives available as solvent-free liquids. This sets them apart from conventional linear comb copoly-mers of comparable molecular size.8
Branched, spherical core-shell copolymers are soluble in and compatible with highly polar formulations, as well as in medium and nonpolar formulations. These wetting and dispersing agents are suitable for a wide range of pigments from inorganic particles to organic pigments and carbon blacks. Figure 17 shows the viscosity reduction of grinds of several organic pigments as well as a carbon black in a universal grinding resin. The viscosity was measured at a shear rate of 1/s using cone/plate geometry equipment (25 mm, 1° at 23 °C) 24 hrs after grinding. The core-shell additive (Additive 2) generates a significantly stronger viscosity reduction with the chosen organic pigments than the linear additive (Additive 1).
There are many additional advantages of core-shell polymers. One example is the application of amino-functional additives in a reactive resin system. Traditional amino-functional additives have limited usability in reactive systems such as epoxy resins because of their lower storage stability and shorter pot life. Branched, spherical polymer structures tailor made for reactive formulations can be used in reactive formulations without exhibiting any of the aforementioned negative side effects. Figure 18 compares the storage stability of a standard amino-functional wetting and dispersing additive (Additive 1) and a core-shell copolymer amino-functional wetting and dispersing additive (Additive 2). Both were added at 3.5 %wt solid on resin to an epoxy base.
The addition of the standard additive to the epoxy base greatly increases flow time within hours and finally, gelling. The exposed amino groups in additives of structure type 1 and their catalytic activity towards epoxy curing are the reason for the low storage stability. In contrast, a stable viscosity over the entire storage period of 12 weeks is achieved with an additive with a core-shell copolymer structure.
As another example, the performance of branched, spherical additives in a reactive aliphatic-aromatic isocyanate-based curing agent was evaluated. The pot life of an isocyanate-based formulation is considerably shortened when a standard amino-functional additive is present. However, the use of a core-shell additive does not have a significant impact on the pot life. The sterically shielded amino groups, as present in a core-shell additive, are much less prone to catalyze urethane formation.
Modern core-shell additives present many additional benefits. Figure 19 shows that compared with the initial amount of 37.5 %wt of aluminum trihydrate (ATH), the use of a core-shell additive makes it possible to increase the flame retardant concentration to 52.5 %wt at constant resin viscosity. That result highlights the very broad field of application for branched, spherical wetting and dispersing agents. These core-shell copolymers are effective with a large array of organic pigments as well as inorganic fillers, and show outstanding performance even in reactive systems without generating undesired side effects such as shorter pot life, gelling, longer curing reactions or decreased crosslinking density.
Conclusion
Wetting and dispersing additives have been subject to permanent change and adaptation over the past few decades. The first additives were optimized to disperse inorganic pigments in organic solvents. The next requirement was the wetting and dispersing of organic pigments in organic solvents. New environmental awareness led to the development of waterborne systems for which completely new systems had to be developed. High-performance applications necessitated the introduction of controlled polymerization techniques to obtain polymers with very low polydispersity. Another driver for new developments is ever-increasing VOC requirements. Finally, additional knowledge about the toxicity of some solvents, but also public acceptance force new developments to meet changing requirements.
Systems that were state of the art just a few years ago have become obsolete technology for some applications due to changes in legislation or market requirements. So, we feel that only continuous, focused R&D in the development of wetting and dispersing additives will ensure that the current and future needs of our customers can be met.
References
1 Du, J.W. Surfactants, Dispersants and Defoamers for the Coatings, Inks, and Adhesives Industries. Coatings Technology Handbook (3rd Edition) 2006, 75/1-75/13.
2 Sato, T. Assessment of Dispersion. Journal of Coatings Technology 1979, 51 (657), 79-85.
3 Kaluza, U. Flocculation of Pigments in Paints - Effects and Causes. Progress in Organic Coatings 1982, 10 (3), 289-330.
4 (a) Kaluza, U. Flocculation - Which Factors Influence It? Part 4. Pigment & Resin Technology 1980, 9 (12), 3-9; (b) Kaluza, U. Flocculation - Which Factors Influence It? Part I. Pigment & Resin Technology 1980, 9 (8), 4-7.
5 Topham, A. Dispersing Agents for Pigments in Organic Liquids. Progress in Organic Coatings 1978, 5 (3), 237-43.
6 Vernardakis, T.G. Improving Dispersion of Organic Pigments. Modern Paint and Coatings1985, 75 (9), 32-4, 36, 38, 40, 42.
7 (a) Goebelt, B. Controlled Polymerisation Techniques for the Design of Novel Polymeric Additives. FATIPEC Congress 2004, 27th (Vol. 1), 303-312; (b) Goebelt, B. Controlled Polymerisation Techniques for the Design of Novel Polymeric Additives. Pitture e Vernici, European Coatings 2005, 81 (2), 39-45; (c) Krappe, U.; Scholz, W. Living Polymerisation a New Way to New Additives. FATIPEC Congress 2000, 25th (Vol. 3), 101-106.
8 Rudolfi, A.; Krohnen, M.; Piestert, F.; Mossmer, S. Designed for Selective Adsorption: Dispersants Based on Core-Shell Structures Stabilize Pigments in Reactive Systems.European Coatings Journal2013, (11), 22-26.
By Dr. Horst M. Sulzbach, Dr. Michael Bessel and Janos Hajas, BYK-Chemie GmbH, Wesel, Germany
This paper was presented at the 2016 Waterborne Symposium in New Orleans.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!




