Dispersant Technology for Red and Yellow Iron Oxides
A New Additive Technology for Waterborne Iron Oxide Pigment Concentrates

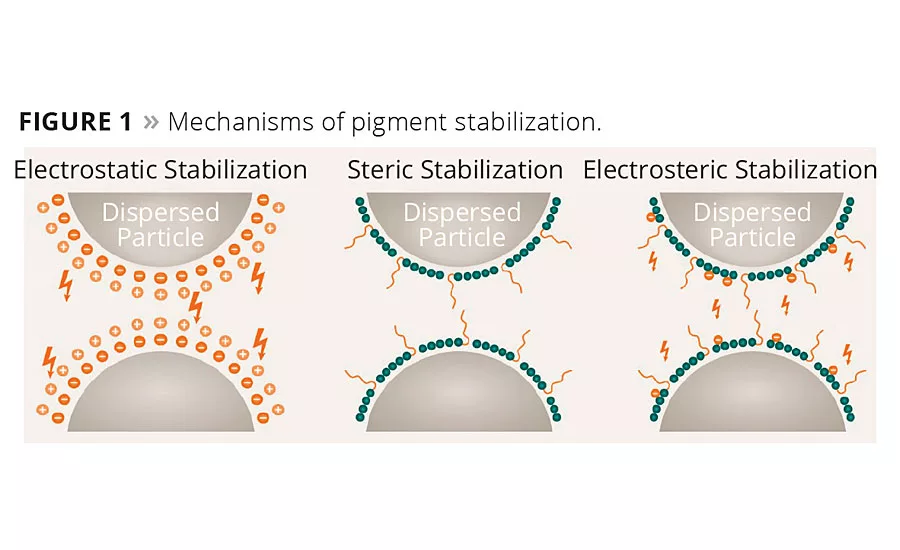
Figure 1
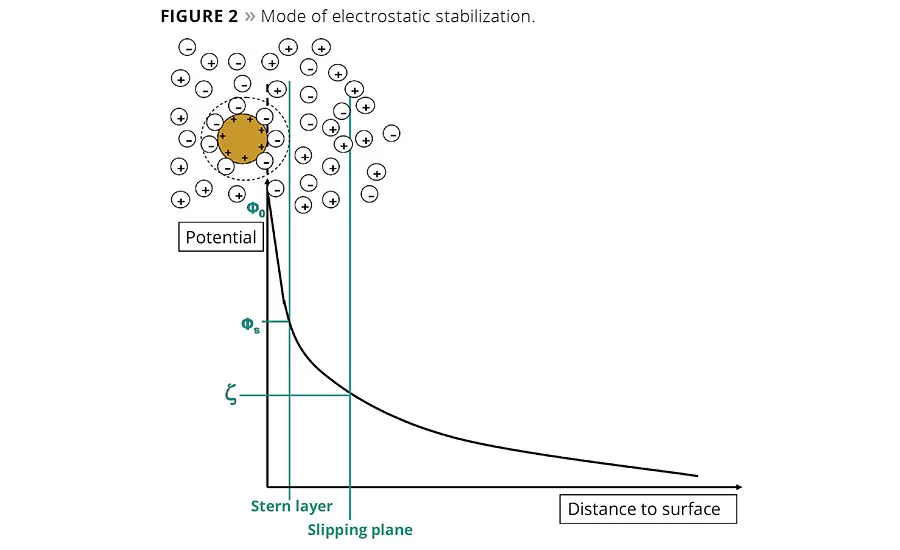
Figure 2
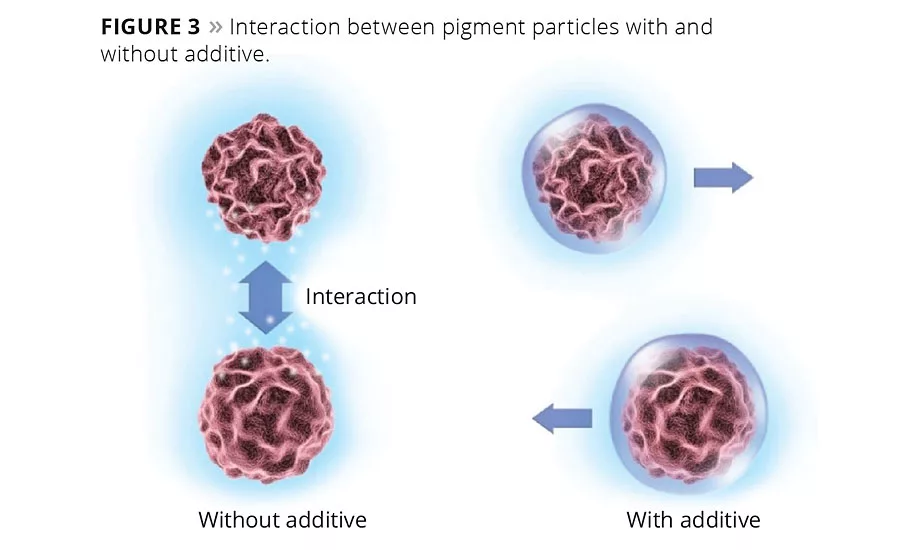
Figure 3
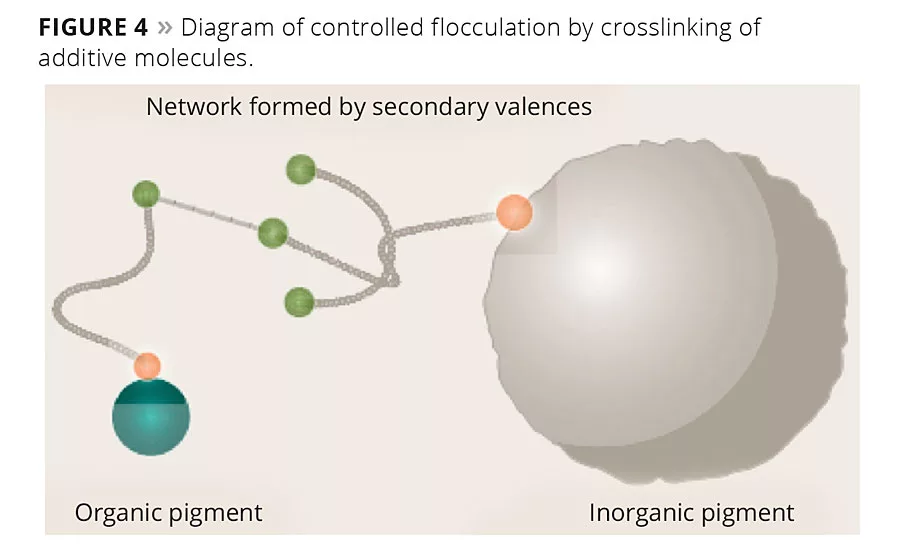
Figure 4
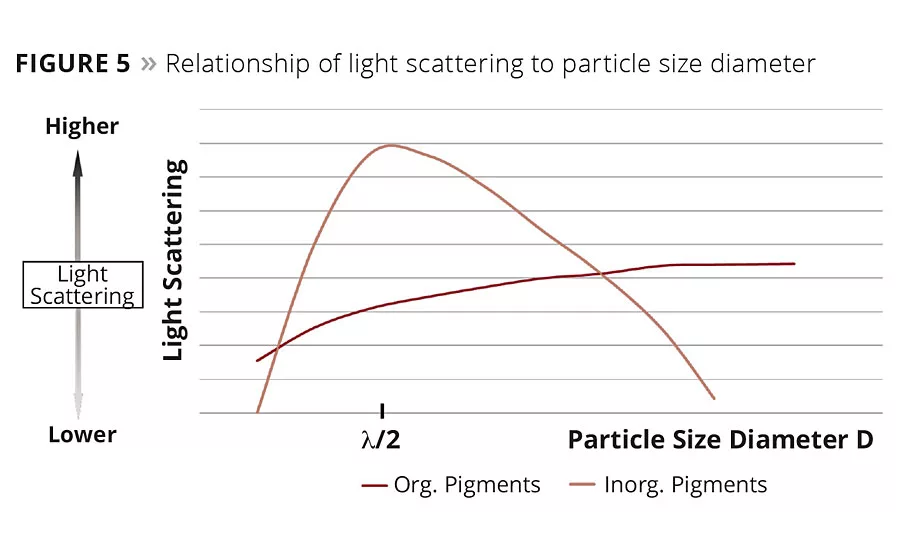
Figure 5
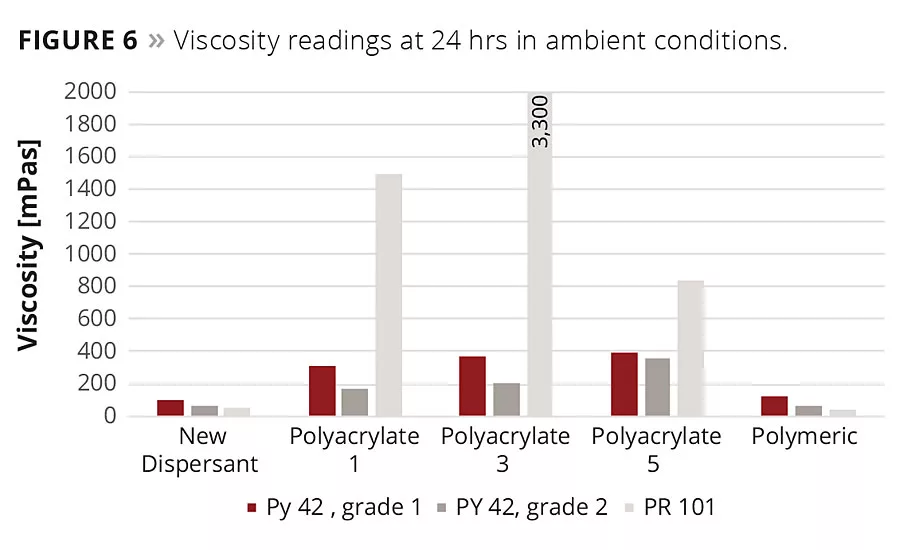
Figure 6

Figure 7
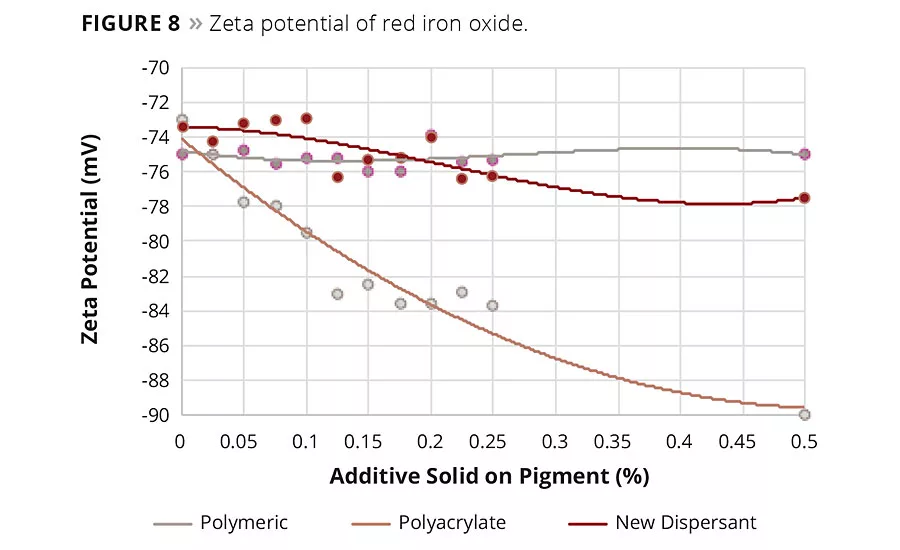
Figure 8
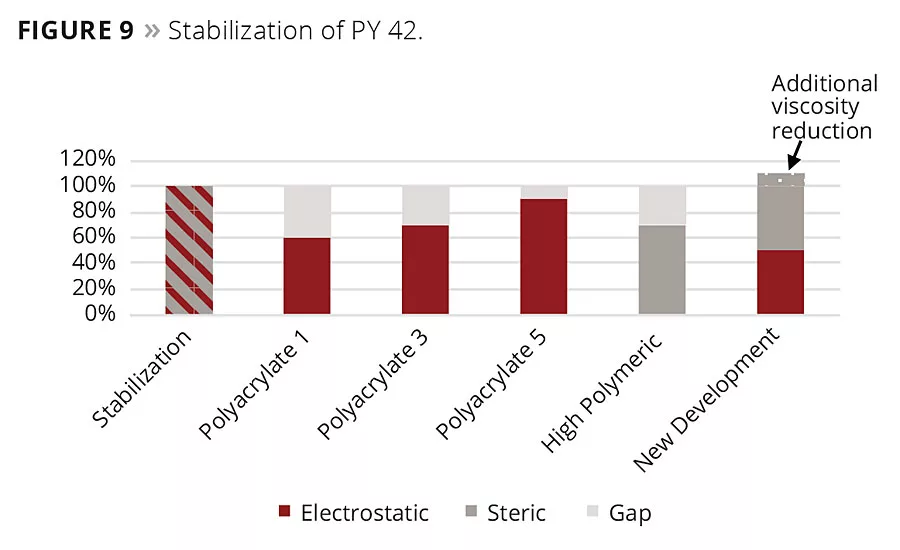
Figure 9
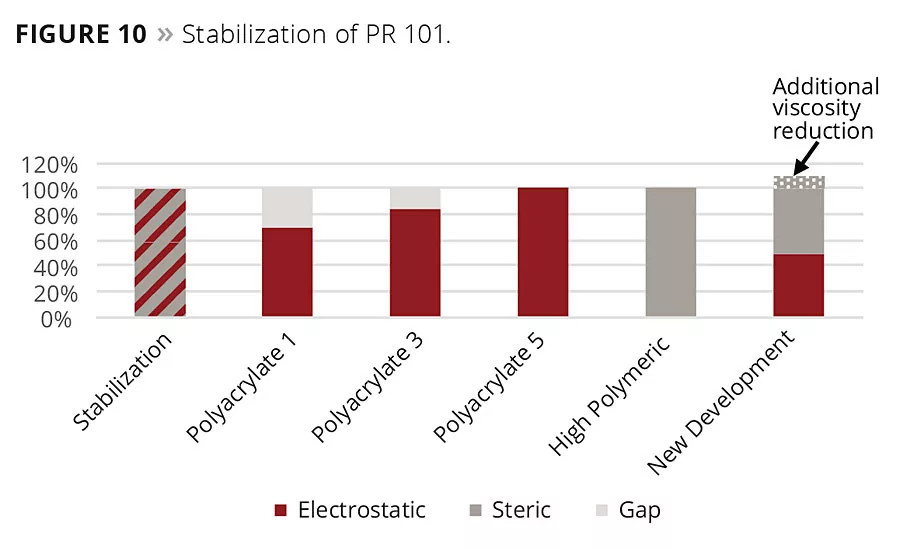
Figure 10
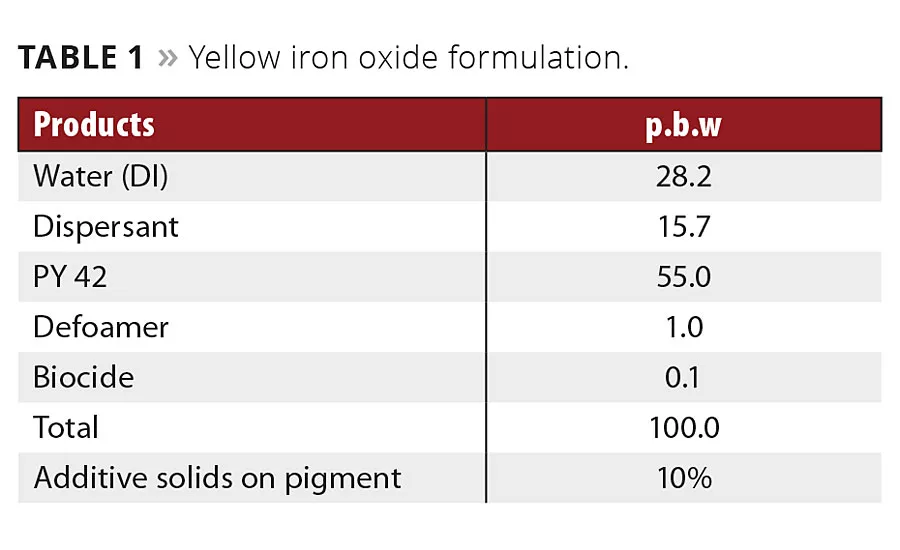
Table 1
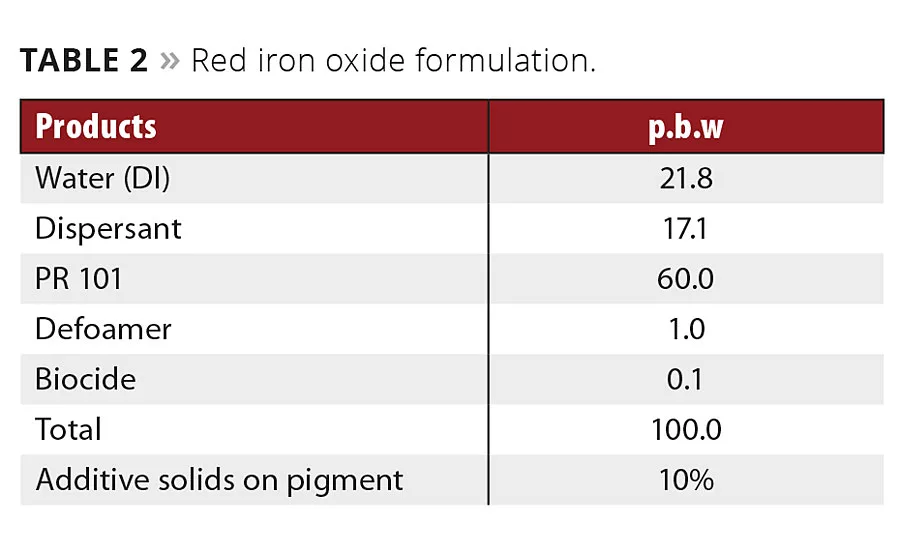
Table 2
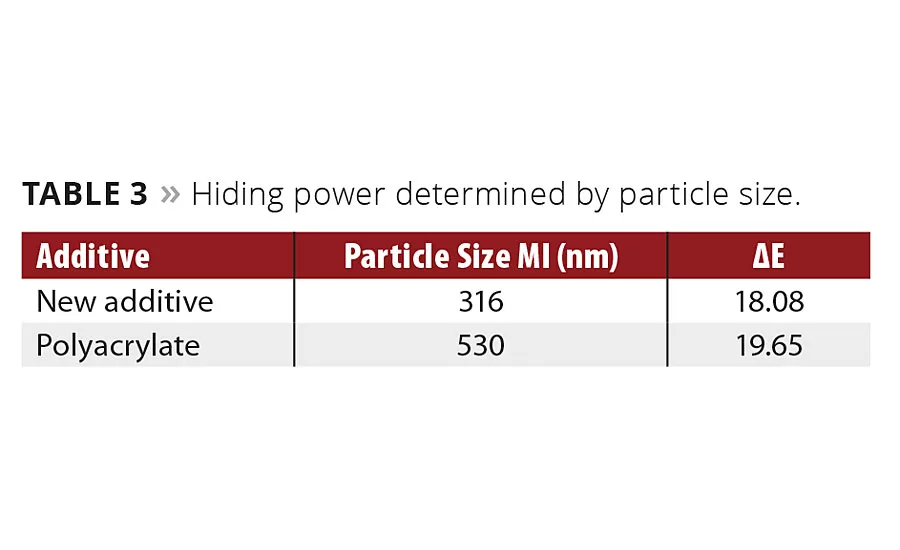
Table 3
Pigment concentrates have always been a modern and flexible way to produce colored paints. Especially in waterborne applications, these concentrates have to be suitable for a broad range of different binder technologies. Because of that, the concentrate formulations are usually free of binders and offer a broad compatibility. Furthermore, the demands in terms of storage stability, color strength and cost efficiency are very high. These demands are addressed directly to the additive technology. The additive is expected to provide outstanding viscosity reduction in order to achieve high pigment loadings and the most economic grind. It has to stabilize the viscosity over long periods of time and has to develop maximum color strength to avoid underutilizing any expensive pigments. Iron oxides are the most important pigment class when it comes to decorative coatings. The difficulty in iron oxides lies in the shape and not the wetting of the pigment. Iron oxide yellow tends to give dilatant rheology, which can be a disaster when automatically dosing in a dispensing machine. On the other hand, iron oxide red tends to increase in viscosity over time in pigment concentrates.
This article explains the different structures of wetting and dispersing additives that could be used in waterborne, binder-free pigment concentrates for iron oxides. The stability and wetting of the pigment concentrates will be reflected by the viscosity reduction, storage stability and coloristic properties. The zeta potential is used for characterizing the wetting behavior and dispersing efficiency. Guidelines for formulating iron oxide pigments will be provided at the end.
Pigment Concentrate Technology
Pigment dispersion is the most important step in the process of producing a colored coating. For waterborne decorative coatings, a white base paint is typically produced by a direct grind, and the color is customized by using a pigment concentrate. These pigment concentrates are usually binder-free to offer better compatibility in a wide range of different base paints. Iron oxides are the second biggest group of pigments in decorative coatings, with the first being titanium dioxide. Large quantities of pigment concentrates are produced with iron oxide red and yellow. These are used for in-house tinting as well as in large tinting machines at the point of sale.
The target for production of iron oxide pigment concentrates is to achieve a maximum pigment loading to reduce the effort in production. The biggest challenge is to stabilize the pigment in the liquid phase and thereby achieve good long-term stability without settling. Pigment dispersion and stabilization are hardly possible without the addition of suitable wetting and dispersing additives. Iron oxides have a very high density, so achieving good stability at a high pigment loading is difficult. It is a tough job, but it is beneficial to have a low viscosity and free-flowing pigment concentrates.
Step One: Pigment Wetting
Pigment dispersion can be broken down into three consecutive steps: wetting, dispersion and stabilization. The first step of the dispersion process is wetting the pigments by a liquid. Proper wetting of pigments is essential for them to be evenly distributed in a liquid. Air trapped in the pigment must be removed and the pigment particle must be fully enclosed by the liquid medium.1 Described by the Young equation (Equation 1), for the pigment surface to be wetted by a liquid, the surface tension of the liquid must be lower than the surface energy of the pigment.1 A liquid with lower surface tension wets pigments better than one that has a high surface tension. Therefore, an additive that promotes wetting must mainly reduce the surface tension of the liquid.
|
Ys = Ysl + Yl • cosΘ |
(1) |
Step Two: Pigment Dispersion
Now that the pigment is wetted, the next step in the process is pigment dispersion. The target of the dispersing process is to achieve very small particle sizes with a large surface area. This leads to high color strength and good hiding power. The additive reduces Van-der-Waals interaction between pigment particles, and in return, this lowers the viscosity of the millbase.2 This allows for higher pigment loading to be reached.
In the dispersion process, the pigment agglomerates are broken down mechanically into primary particles and small aggregates. Energy is required to break up aggregates and agglomerates. To break up agglomerates and increase the surface area (dA), energy input (dW) is needed (Equation 2).1 This energy is proportional to the surface tension (Y). The smaller the surface tension, the greater the surface area will be for a certain amount of energy.2
|
dW = Y • dA |
(2) |
Step Three: Stabilization
The next step in the dispersion process is stabilizing the pigments. Stabilization of solid particles is the ability to keep all solid particles separated at a certain distance and stop agglomerates, aggregates and flocculates. Solid particles in liquid will move around and collide with each other according to Brownian motion. If these particles are not well stabilized they will re-agglomerate and flocculate back together. To achieve a good stabilization of the pigments, the wetting and dispersing additive has to adsorb on the surface of the pigment. Therefore, the additive has to have anchor groups with high affinity to the pigment surface. For iron oxide pigments, the additive should have affinity groups that are able to build hydrogen bonding or dipole-dipole forces with the surface of the pigment. The most suitable functional groups for a good adsorption on iron oxides are hydroxyl, carbonyl or carboxyl groups.
The stabilization of the pigments can be achieved by the following mechanisms (Figure 1):
- Electrostatic stabilization;
- Steric stabilization;
- Electrosteric stabilization.
Electrostatic Stabilization and Zeta Potential
The most important stabilizing factor in waterborne formulations is electrostatic repulsion. The most ideal case is when the wetting and dispersing additive adsorbed onto the pigment surface dissociates into an anionic and cationic part. The cationic counter ions form a mobile diffuse cloud around the pigment particle, which leads to an electrostatic double layer.1 With a strong double layer, repulsion dominates and the dispersion is stable.
Electrostatic stabilization can be quantified by the zeta potential, z, which is a measure of the potential at a shear layer in a dispersion. The first adsorption layer with negative charges is generated by the wetting and dispersing additive, but not the whole charge of the pigment particle is compensated. A second layer with a diffuse charge distribution is built up predominantly with counter ions. Both layers represent the electrostatic double layer, known as the ion cloud.3
The zeta potential is measured because the surface potential cannot be directly determined. In this case, the migration speed of the particles in an electrical field is evaluated. When electrostatic charged particles move in an electrical field, they take a part of the ion cloud with them. The higher the distance of the ions to the pigment surface, the lower the interaction is with the pigment surface.3
The loosely bound diffuse layer shears off, and the potential at this shear plane is termed zeta potential. The higher the zeta potential, the better the stabilization of the pigments (Figure 2). As the zeta potential approaches zero, the tendency of the particles to agglomerate increases.
The zeta potential does not describe the steric stabilization, which is another vital mechanism in waterborne formulations. Steric stabilization is not achieved by ions and therefore potential cannot be measured.
Steric Stabilization
In contrast to electrostatic stabilization, polymeric side chains are necessary for steric stabilization. The side chains append themselves to the pigment surface and warrant adsorption of the additive. When the pigment particles come closer to each other, the polymeric side chains reduce mobility and lower entropy.
An essential factor in stabilization is the reduction of interactions between pigment particles that would otherwise cause flocculation (Figure 3). These interactions also restrict the movement of the particles and give rise to the viscosity. The better the stabilization, the lower the interactions and therefore the lower the viscosity.
Electrosteric Stabilization
Wetting and dispersing additives have complex demands, therefore it is sometimes useful to combine electrostatic and steric stabilization. This mechanism is known as electrosteric stabilization. Electrosteric additives have the ability to fulfill high demands for stabilization and durability.
Electrosteric additives prevent pigment particles from approaching each other by “controlled flocculation” (Figure 4). The molecules of the additive interact with each other and the pigment surface, forming a three-dimensional network.1 Floating of pigments is a result of different pigment mobility. With electrosteric stabilization, pigments bind to flocculates of the same color and prevent floating in the dispersion.
Results and Discussion
Typical formulations of waterborne binder-free pigment concentrates for iron oxide yellow and iron oxide red were used to prepare different pigment concentrates (Tables 1 and 2). Three different polyacrylate salts, one high polymeric additive and a new additive were tested.
Particle Size and Hiding Power
The hiding power of pigment particles determined by light scattering is related to the particle size. Inorganic pigments show a maximum in light scattering at a particle size half the wavelength of the scattered light (l/2) (Figure 5).
The prepared colorants were let down with a styrene acrylic emulsion. Drawdowns on black and white charts were prepared. The hiding power was determined by measuring the delta E value between the drawdowns over white and the drawdowns over black (Table 3). The lower the delta E value, the higher the hiding power. The particle size distribution was measured using dynamic light scattering (MicroWave by MicroTrack). With the new additive, the particle size of iron oxide yellow was close to the optimum (l/2). With this particle size, the highest hiding power could be achieved.
Viscosity and Stability
The viscosity of the pigment concentrates was measured 24 hrs after preparation (Figure 6). A cone plate rheometer was used for the measurement. Future work includes viscosity readings over two weeks in an oven at 50 °C.
The polyacrylate additives exhibited useful results with yellow iron oxide in the beginning. The polymeric additive and the new additive displayed a very strong viscosity reduction with the yellow oxide. More diverse results were found for the red iron oxide. Only one of the polyacrylate additives could achieve a processable viscosity. The polymeric and new additive achieved a much lower viscosity.
Zeta Potential
The zeta potential was measured in a 5% pigment slurry. The respective additive was titrated until a constant zeta potential was reached. The highest influence on the zeta potential could be seen with an additive addition up to 0.5% solid on pigment. The lowest zeta potential was achieved with polyacrylates, which reflects that the strongest stabilization is accomplished with electrostatic chemistry. As expected, the high polymeric additive had almost no influence on the zeta potential. This additive class does not provide any electrostatic stabilization. The new additive had a zeta potential in between the polyacrylates and the polymeric additive (Figures 7 and 8).
The zeta potential alone is not sufficient enough to interpret results obtained by viscosity and stability measurement completely. The zeta potential does not give information about the steric stabilization of the pigment particles. Providing that the steric stabilization contributes beneficially to the performance, the results can be interpreted as follows (Figures 9 and 10). To stabilize the iron oxide particles, a certain amount of stabilization energy is needed. In some cases, electrostatic energy cannot stabilize pigments on its own. Sometimes it is necessary to have additional steric stabilization as well.
Conclusion
The results show that the new additive performs on a very high level with a broad range of iron oxide pigments. It combines outstanding viscosity reduction and excellent hiding power. The new additive provides an optimized balance between electrostatic and steric stabilization, making it a very efficient additive. This additive reduces complexity and contributes to a more cost-efficient way to formulate decorative coatings.
References
1 Evonik Corporation, TEGO Chemie Service GmbH. TEGO Journal, 4th ed.; 2012. pp 79-89.
2 Heilen, W. Additives for Waterborne Coatings. European Coatings Literature, Vincentz Network GmbH & Co.: Hannover, Germany, 2009.
3 Winkler, J. Dispergieren von Pigmenten und Füllstoffen. European Coatings Literature, Vincentz Network GmbH & Co.: Hannover, Germany, 2012.
This paper was presented at the 2016 Waterborne Symposium in New Orleans.
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!





