Conventional Microbiology and the Truth
A Paint Preservation Perspective
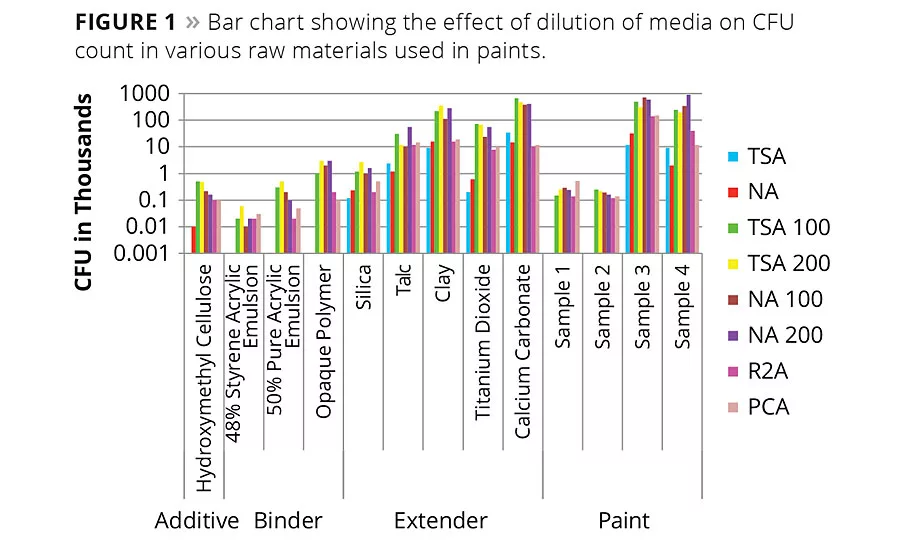
Figure 1
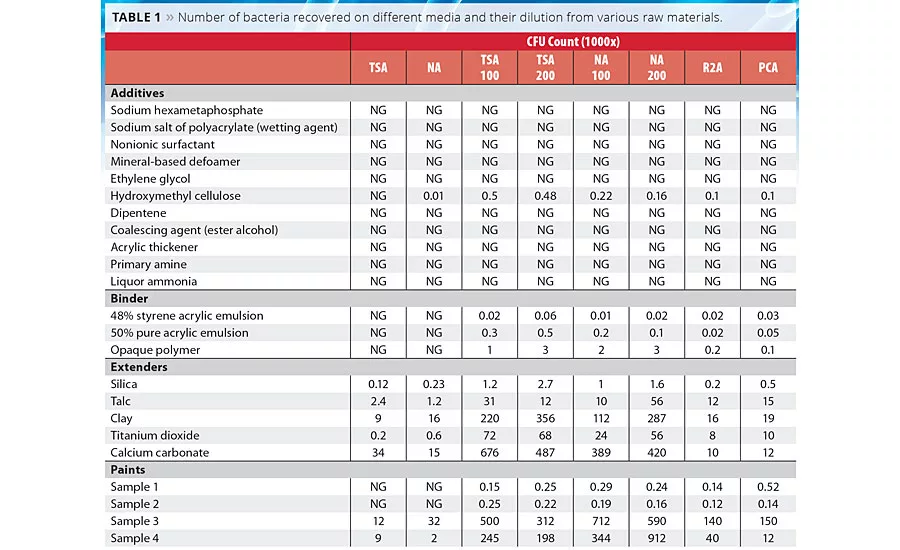
Table 1
Microorganisms are ubiquitous in the environment; they are present wherever there is water.1 They are highly versatile in their nutritional requirements for growth, which can be met by raw materials used to make water-based coatings in their wet stage. The metabolisms of the microbes (bacteria, yeast and molds) pose a potential threat to many industries where water is being used in product manufacture or in process.
In the paint industry it is primarily bacteria, and sometimes yeast, that are responsible for wet-stage spoilage of paint product. Microbial spoilage can be observed as a foul odor, gelation, settling, curdling and blackening.2 Identified groups of organisms responsible for spoilage of wet-stage paint are normally found in water and soil, as well as in natural raw materials like clay, pigments, TiO2, etc. These microorganisms are mainly members of the Enterobacteraceae family, such as the Citrobacter, Enterobacter and Escherichia species, and Pseudomonadaceae, especially Pseudomonas spp. being the most frequently encountered contaminants. Later spoilage with anaerobes such as Desulfovibrio spp. give badly infected products a characteristic odor and in many cases discoloration.3
It is a commonly used practice to count colony forming units (CFU) to determine the severity of microbial contamination. A CFU count is done with the help of commercially available dip slides, nutrient media or soyabean casein digest media. These media, which are used widely in the paint industry, are nutrient rich and support the growth of a few types of microorganisms (belonging to six major phylum of the bacterial world out of 60-80 known groups). These high-nutrient media generally do not support the growth of many other bacteria, therefore the presence of those bacteria is overlooked.4 There are reports suggesting that the growth of more diverse type of bacteria can be improved just by diluting the nutrient concentrations in media.5 This article proposes that dilution of media (100 times, 200 times and 500 times) or moderately nutrient-rich media (R2A and R3A) support more numbers and types of bacteria on the solidified media than traditionally used nutrient-rich media without dilution. To support this hypothesis, 19 raw materials used in water-based architectural paint and four water-based decorative paint samples (6-12 months heat aged) were considered for this study.
Materials and Methods
Paint Samples
Water-based decorative paint samples aged at 40 ˚C for six months (paint samples 1 and 2) to 12 months (paint samples 3 and 4) were collected from various sources.
Paint Raw Materials
Additives, extenders and pigments, thickeners and strainers were collected from various sources. The following raw materials from various sources were taken for this study: sodium hexametaphosphate, sodium salt of polyacrylate (wetting agent), nonionic surfactant, mineral-based defoamer, ethylene glycol, triethylene glycol, hydroxymethyl cellulose, di-pentene, coalescing agent (ester alcohol), acrylic thickener, 48% styrene acrylic emulsion, 50% pure acrylic emulsion, opaque polymer, primary amine, liquor ammonia, silica (10 micron), talc, clay, calcium carbonate, titanium dioxide (R-902) and titanium dioxide (CR-828).
Media
Tryptic soy agar (TSA) and nutrient agar (NA) were used as nutrient-rich media. Commercially available R2A and R3A were used as moderately nutrient-rich media. TSA and NA media were diluted 100 and 200 times with water and solidified with agar to make nutrient-poor media TSA 100, TSA 200, NA 100 and NA 200 respectively.
Culture Condition and CFU Count
The CFU count was taken by serially diluting the paint sample by 1000 times, and 100 µl of it was placed on each media and spread evenly by a sterilized glass spreader. The plates were incubated in an upright position at 30 ˚C for 24 hrs to one week. Petri plates were wrapped with parafilm to minimize dehydration of the media under incubation. Colonies were counted manually every 24 hrs for seven days to enumerate the growth of fast-growing bacteria as well as slow-growing bacteria. Counting was done by using permanent marker pen of different colored ink to avoid any overlapping in the previous day’s count.
Results
It was observed that, in general, diluted media recovered a higher number of cells than undiluted media both in paint and in raw materials used in the manufacture of paints. There was a 10- to 100-fold increase in the number of colonies developed compared to traditionally used nutrient-rich media (TSA, NA) (Table 1). It was observed that moderate nutrition-containing media like R2A and PCA also helped to increase the CFU number on solid media. Although there was a fold shift observed upon 100-fold dilution, at 200-fold dilution the number of recovered bacterial colonies only changed minimally. Interestingly, the 50% pure acrylic emulsion, opaque polymer and 48% styrene acrylic emulsion showed no colonies on nutrient-rich media, while the same sample showed 2-3 log increase in CFU count upon diluted media. Extenders and paints also showed similar patterns. Paint samples 3 and 4 showed a growth of 3-4 log increase in CFU count even in nutritionally rich media, probably because this paint was manufactured six months prior to this study. Among all the extenders, only silica and talc did not show any log level difference in bacterial growth compared to nutritionally rich media. The reason may be because the diversity of bacteria was very poor in these samples.
Discussion
In microbiology, the terms ‘viability’ and ‘cultivability’ are often associated. Many bacteria respond to various environmental stresses by entering into a novel physiological state where the cells remain viable, but are no longer cultivable on standard laboratory media and are referred to as ‘viable but non-cultivable’(VBNC), whereas cultivability clearly depends upon the medium used.
A nutrient-rich condition is one of the major reasons for not detecting VBNC in traditional medium like TSA and NA. Nutrient-rich media may inflict shock to the bacterial cells, and therefore existing enumeration techniques underestimate the microbial load and diversity.6
In 2012, Azevedo et al. reported that Sphingomonas capsulata, Methylobacterium sp., E. coli CECT 434 and Pseudomonas fluorescens ATCC 13525 showed higher average improvement of recovery when cells were exposed to the water compared to R2A (low-nutrient media) and TSA (high-nutrient media).7 Reasoner and Geldreich (1985) concluded in their studies that R2A showed significantly higher bacterial counts than other nutrient-rich traditional media.8 In 1996, Jensen et al. established that the cultivability of marine bacteria can be dramatically improved by the use of low-nutrient media.9
Similarly, in this study a higher number of cells were recovered from a diluted, low-nutrient medium than from the undiluted, nutrient-rich medium. In this study it was also reported that moderate-nutrition media like R2A and PCA showed better recovery of bacteria than traditionally nutrient-rich media (TSA, NA). In the case of the 50% pure acrylic emulsion, opaque polymer and 40% styrene acrylic emulsion, bacteria could not be detected on normal nutrient-rich media, whereas up to 3 log growth was reported on diluted media. Among the tested extenders all showed a 10-100 log level difference in bacterial growth compared to nutritionally rich media, whereas in paint samples 1 and 2 not much of a difference was observed, perhaps because the diversity of bacteria was very poor in these two samples.
A VBNC state is of great concern because bacterial cells can display enhanced resistance to antimicrobial agents (mainly due to low metabolic activity) and retain their virulence after resuscitation.10 Preservatives are widely used to protect emulsions and paint from microbial attack. During manufacturing, VBNC bacteria from various raw materials may lead to miscalculations of correct and appropriate amounts of biocidal doses. Also, these ‘hidden’ bacteria can develop a tolerance to these preservatives and might be a potential threat to the paint’s shelf life .
References
1. Hurst, C.J. Neighborhood and Community Involvements: No Microbe is an Island. Manual of Environmental Microbiology, edited by C. J. Hurst. 2nd ed., pp. 6-8, (2001) Washington DC; American Society of Microbiology.
2. Paulus, W. Directory of Microbicides for the Protection of Materials: A Handbook, 2005th Ed. Netherlands. Springer (2005).
3. Gillatt, J. The Microbiological Spoilage of Emulsion Paints During Manufacture and Its Prevention. Paintindia, 2011 61: 5, 52-54.
4. Handelsman, J. Metagenomics: Application of Genomics to Uncultured Microorganisms. Microbiology and Molecular Biology Reviews, 68(4), 669-685, (2004).
5. Connon, S.A.; Giovannoni, S.J. High-Throughput Methods for Culturing Microorganisms in Very-Low-Nutrient Media Yield Diverse New Marine Isolates. Appl. Environ. Microbiol. 2002; 68:3878-3885.
6. Staley, J.T.; Konopka, A. Measurement of In Situ Activities of Nonphotosynthetic Microorganisms in Aquatic and Terrestrial Habitats. Annu. Rev. Microbiol. 1985; 39:321-346.
7. Azevedo, N.F.; Bragança, S.M.; Simões, L.C.; Cerqueira, L.; Almeida, C.; Keevil, C.W.; Vieira, M.J. Proposal for a Method to Estimate Nutrient Shock Effects in Bacteria. BMC Res Notes. 8;5:422, (2012).
8. Reasoner, D.J.; Geldreich, E.E. A New Medium for the Enumeration and Subculture of Bacteria from Potable Water. Appl Environ Microbiol. 1985; 49(1):1-7.
9. Jensen, P.R.; Kauffman, C.A.; Fenical, W. High Recovery of Culturable Bacteria from the Surfaces of Marine Algae. Mar Biol. 1996; 126:1-7.
10. Oliver, J. D. Recent Findings on the Viable but Nonculturable State in Pathogenic Bacteria. FEMS Microbiol. Rev. 34, 415-425, (2010).
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!







