A Greener Solution to Deter Marine Biofouling

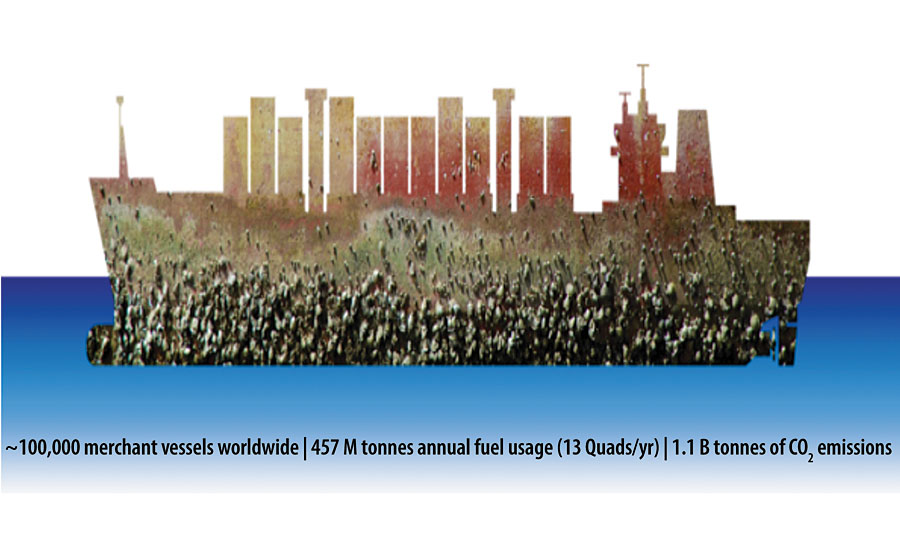
The process of growth and accumulation of barnacles, mussels, algae and various organisms on ship hulls and other submerged structures, known as marine biofouling, is a long-standing problem closely related to the efficiency and safety of human activities and properties in marine environments. The problem is ubiquitous and can be easily found on large cargo vessels, commercial fishing boats, naval vessels, recreational yachts and small craft, aquaculture gear, ocean sensors, UUVs, marine hydrokinetic structures, and so on. The adverse effects of marine fouling on these properties include a significant increase in hydrodynamic drag and associated additional fuel consumption, and increased emissions, corrosion and damage to the structure, spreading of non-indigenous species and diseases disturbing the marine ecosystems and causing significant economic loss. It is estimated that heavy calcareous fouling on ship hulls such as barnacle attachment can reduce fuel efficiency by up to 85%. 1,2 Even light (or heavy) slime coverage can cause about a 9% (or 17%) increase in total hydrodynamic resistance, which can cause up to 18% shaft power penalties. 1 Approximately 2% (~13 Quads) of the world’s energy is currently used in the commercial marine shipping industry, consisting of nearly 100,000 commercial cargo ships, which also contributes to 1.1 billion tonnes of carbon emissions. 3 To put this in a different context, globally $60 billion/yr in fuel cost alone can be saved if we can successfully address the marine biofouling problem on ship hulls (Figure 1). 4
Traditional solutions to address the hull fouling problem typically involve the application of toxic substances (biocides) to kill the organisms. The rise of self-polishing paints containing tributyltin (TBT) in the late 1970s seemed to have permanently solved this long-standing problem. They were highly effective in maintaining a clean hull for a long enough time. Nevertheless, these highly toxic tin compounds resulted in widespread environmental harm from damage to non-target organisms and surrounding ecosystems, and were gradually phased out. Eventually, the International Maritime Organization (IMO) placed a global ban on TBT-based paints in 2008, forcing the paint manufacturers to go back to copper-based compounds such as cuprous oxide (Cu 2 O) or copper pyrithione. Despite the advancement of the self-polishing copolymer (SPC) binder technologies to control the release rate of copper-based biocides, these paints are less effective than TBT-based paints due to the reduced toxicity of copper. Moreover, recent studies show growing resistance to copper from an increasingly prevalent species in the United States such as Balanus amphitrite (barnacle). 5 With increasing awareness of the environmental impact of toxic chemicals and microplastics leaching from marine paints and due to documented negative effects of copper biocides on the marine environment, there is mounting regulatory pressure from U.S. federal and state agencies to reduce the use of such toxic coatings in the market today. In 2017, Washington State passed a ban to limit the use of copper-based paints on recreational vessels in significant part due to their concern over the effect on salmon aquaculture, making the development of an alternative, non-biocidal solution even more important.
The most widely adopted approach to enable non-biocidal paint is using the fouling release (FR) effect. 6 An engineered surface that would allow only weak adhesion or settlement of organisms can easily release the fouling organisms by the weak shear force created by the movement of a ship. Robert E. Baier established an empirical correlation between the adhesion of fouling organisms and the critical surface tension, widely known as the Baier curve, as shown in Figure 2. 7,8 There is a dip at the critical surface tension of 22-24 mN/m where naturally the lowest amount of marine biofouling has been observed. This value is close to the dispersive component of the surface energy of water and matches well with the surface energy of silicones or polysiloxanes. In other words, the thermodynamic energy penalty to create an interface between silicones and water can be minimized, for example, when a fouling organism is removed from silicone surface and the water is re-wetting the surface.
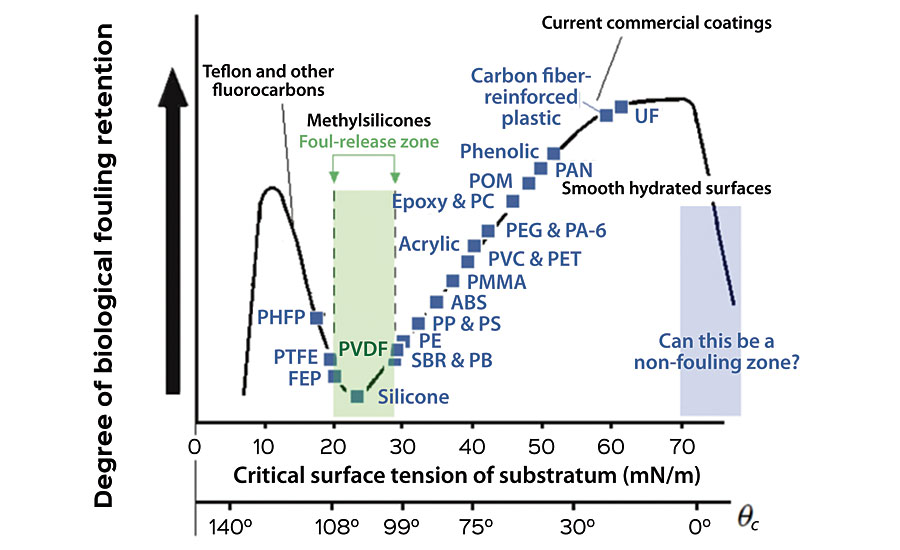
Based on this indicator, several marine coatings companies have developed silicone-based foul release (FR) bottom paints. While these paints effectively reduce the adhesion of more problematic species such as barnacles and mussels, the hydrophobic nature of the surface attracts the settlement of other organisms such as diatom algae (e.g. Navicula incerta), resulting in continuing slime growth on the hull, which still causes a significant level of hydrodynamic drag and invites the settlement of other large fouling organisms if not cleaned.
To overcome this issue, new approaches incorporating chemical moieties known to have very low protein adsorption have been introduced. 6, 9, 10 It is widely known that hydrophilic, charge-neutral and hydrogen bonding-accepting chemical functional groups (e.g. polyethylene glycol (PEG) or zwitterionic moieties) can effectively reduce the adsorption of proteins or small molecules. Various explanations have been proposed including the steric effect, the formation of a tightly bound hydration layer causing additional energy penalty for the proteins to displace the bound water molecules to adhere to the surface, the entropic penalty for the PEG chains to adopt more confined configuration with the departure of water molecules, and others. 9 All of these explanations involve the surface-bound water molecules that must be displaced. The Baier curve also suggests this special feature of water or hydrated surface as another dip at critical surface tension close to that of water. When these chemical moieties are introduced in a silicone FR system, the coating surface becomes heterogeneous with micro-phase separated domains, which are believed to discourage the settlement of marine organisms by providing an ‘ambiguous’ surface.
Despite the long development efforts over the past two decades, the widespread adoption of FR coatings still requires a few remaining challenges to be overcome: 1) the foul release mechanism requires the ship to be moving, which is not always the case (highly dependent on the usage profile) and therefore it is essential to also have biofouling-resistant performance under a static condition; 2) not all organisms can be released at low speed, in particular the release of slime below 10 knots is difficult to achieve; 3) the application of silicone-based paints poses new operational and maintenance challenges at boatyards and dry docks because silicone contamination can cause failure of other types of paints (e.g. fisheyes and blistering of above-the-waterline coatings), and therefore requires quarantining the procedures and adds additional costs and time. Other less significant challenges include the perceived inferior mechanical durability of silicone-based materials compared to other conventional coatings such as epoxy, relatively expensive raw materials, the requirement of tie-coat to ensure adhesion of the silicone-based topcoat, and relatively complicated procedures for repairing and re-applying the topcoats compared to traditional paints.
As an effort to overcome the most challenging issue of foul-release paint (i.e. the performance in static conditions), hybrid coating products combining FR technology and biocides (e.g. copper pyrithione and Selektope™) have emerged and are gradually gaining market traction. However, such a solution would still not be fully environmentally friendly and sustainable due to the use of leaching biocides. 11 Other emerging non-coating-based approaches include: 1) light-generated peroxides to discourage fouling, but this is only effective on surfaces with abundant sunlight (e.g. waterline) and becomes less effective with increasing depth; 2) micro-patterned surfaces such as Sharklet™ and microfiber-based hairy surfaces such as Finsulate™, but the performance of using this type of technology alone still largely falls behind conventional biocidal approaches; 3) UV LED illumination to deter fouling, but there are questions about logistics to install and use them on ship hulls, the long-term effects such as development of resistance and mutation, and the side-effects on neighboring ships; 4) grooming of hulls, potentially using a robot combined with FR coatings rather than cleaning, which has to overcome the pressure of additional infrastructure development, long time for adoption, and the emergence of tolerant species.
The SLIPS Approach and the Lessons Learned
Adaptive Surface Technologies, Inc. (AST) develops disruptive repellent coating products addressing high-value, complex, difficult-to-solve and long-standing problems. AST utilizes a revolutionary patented technology that relies on the concept of Slippery Liquid-Infused Porous Surfaces (SLIPS). This Harvard-invented technology is an example of bio-inspired engineering based on Nepenthes, a kind of carnivorous pitcher plant that captures prey using its extremely slippery rim (peristome) of the pitcher on which small insects and animals hydroplane into the deadly trap. SLIPS ® , a synthetic mimic of Nepenthes, provides a broadly repellent surface to some of the most insidious liquids and biofouling by way of a self-healing, extremely smooth and lubricious liquid interface shown schematically in Figure 3. 12 Foulants and contaminants can stick and smear on solid surfaces due to microscopic roughness acting as pinning points, while on SLIPS materials these contaminants simply slide off due to the ultra-smooth nature of stabilized liquid on the surface, which is often chosen to be immiscible with the foulants. 13-16
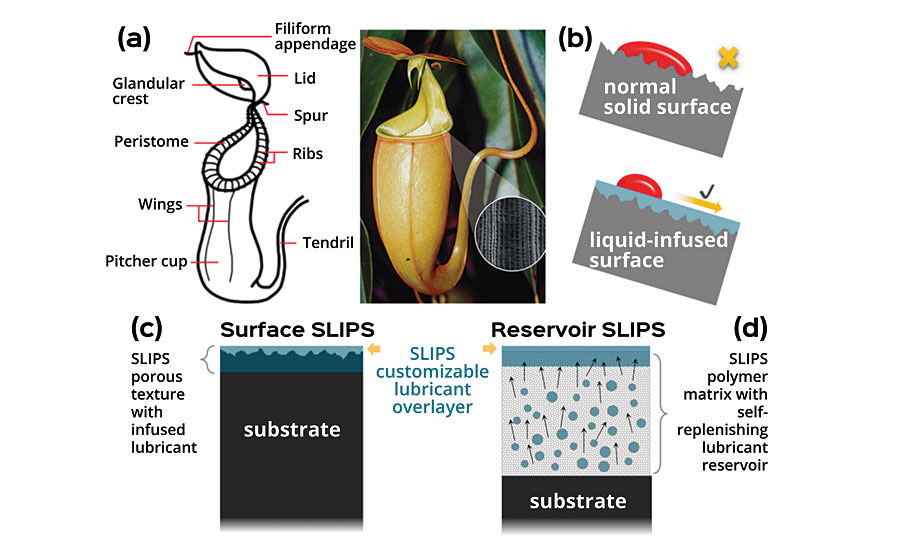
AST developed two technical manifestations of SLIPS to create a stabilized liquid interface, a unique feature of all SLIPS products, as shown in Fig 3(c, d). The Surface SLIPS utilizes a strong capillary force and matched chemistry to thermodynamically stabilize the liquid layer, which is often applied as a spray-on coating. The Reservoir SLIPS combines a curable polymer mixture with a lubricant by carefully designing the polymer mesh size (i.e. the free space formed by a crosslinked network of polymers) and miscibility of the components such that the lubricant can spontaneously migrate to the surface and form a self-replenishing lubricious interface from the reservoir inside the cured polymer system. 17, 18
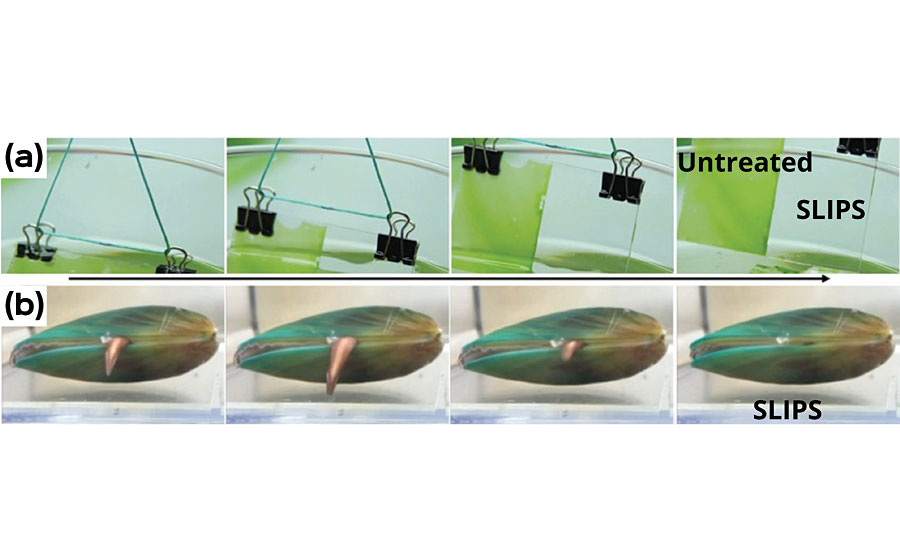
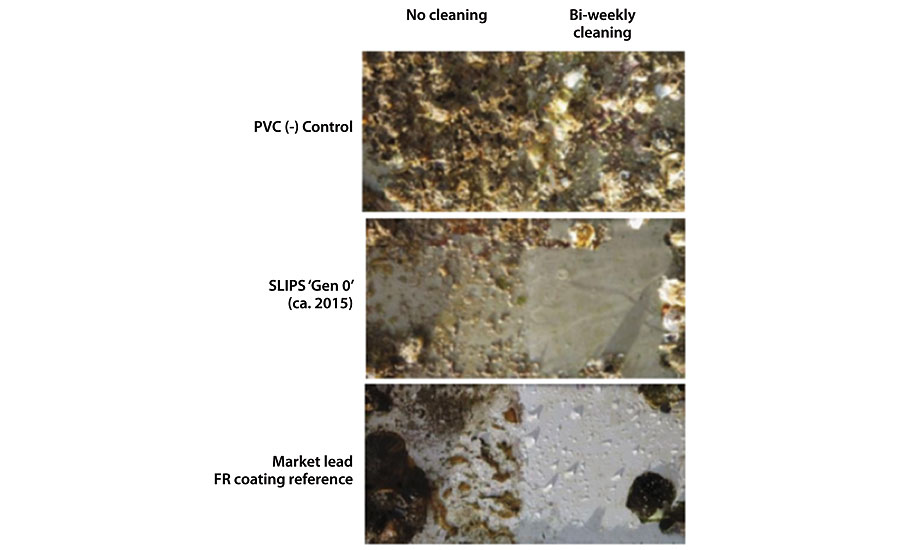
Initially, a 100% silicone-based Reservoir SLIPS system was created and tested for its efficacy in repelling biofouling. 17-20 It was found that such a system is great at repelling some hard fouling species (barnacles and mussels) as well as releasing soft fouling (algal biofilm) even with a very weak shear force owing to the smooth interface between the formed biofilm and the SLIPS surface (Figure 4). 19-21 However, it suffers from slime fouling under long-term static conditions. Over 2.5 years of field testing in Singapore confirmed that this system performs comparably to the best market available FR coating system, though its best benefits are obtained with periodic cleaning, owing to its very easy-to-clean property (Figure 5). However, the requirement for regular cleaning is still non-ideal for most applications. Therefore a fundamentally new approach was necessary to develop a coating effective even under static conditions.
Modular and Hybrid Approaches to Solve the Puzzle
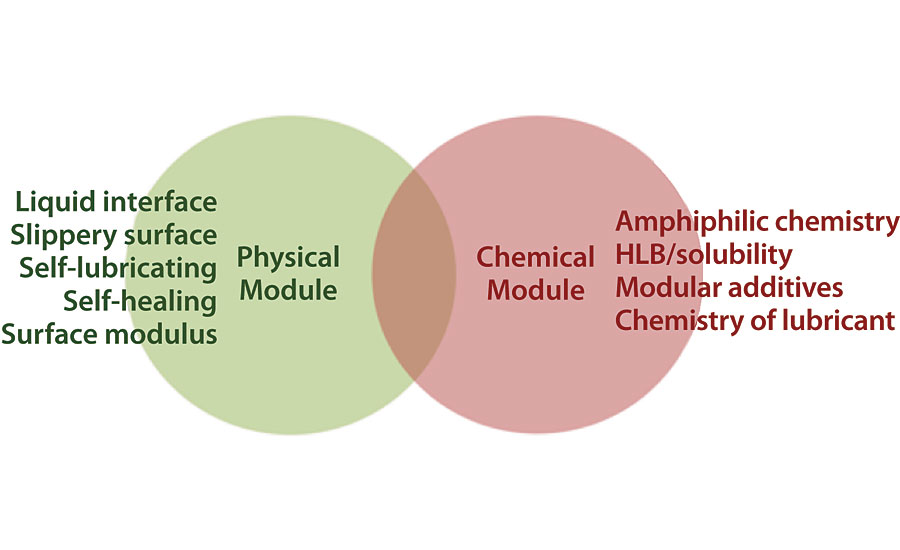
Natural antifouling surfaces generally exhibit both physical and chemical attributes. 9 While there are a variety of synthetic surface chemistries with promising antifouling properties, there is no single chemistry that has been shown to work as an effective universal antifouling strategy. Therefore, a combined approach of using both physical and chemical antifouling strategies is necessary to produce an optimal coating. One such approach would be combining the SLIPS effect with surface chemistries known to minimize biofouling. Going back to the Baier curve where there are two dips of critical surface energy with minimal biofouling, it becomes obvious that a strategy presenting hybrid surface chemistry would be necessary to deter all kinds of marine organisms without killing them. To achieve this goal, AST adopted a modular approach by combining a Physical Module (slippery surface to repel and make it easy to release biofouling – the SLIPS effect) and a Chemical Module (amphiphilic surface chemistry delivered via carefully designed additives) as shown in Figure 6.
Now, the practical question becomes how one can smoothly combine the two approaches in a coating system that can be manufactured cost effectively and on a large scale. In order to incorporate hydrophilic moieties (e.g. PEG or zwitterionic groups) in generally hydrophobic polymer matrices such as silicones, custom molecules would have to be designed and synthesized with the following properties: 1) stable in ambient condition and silicones; 2) can be formulated into silicone systems without prematurely curing or gelating the system; 3) can self-stratify to the interface to deliver amphiphilic chemistry such that the amount added can be kept minimal for cost and for retaining the paint properties; 4) dynamically present hydrophilic moieties when submerged in seawater; 5) compatible with the lubricant in the system to maintain the SLIPS effect for a long period of time.
AST has taken the approach of designing and synthesizing a surface-active polymer (SAP), as shown schematically in Figure 7.
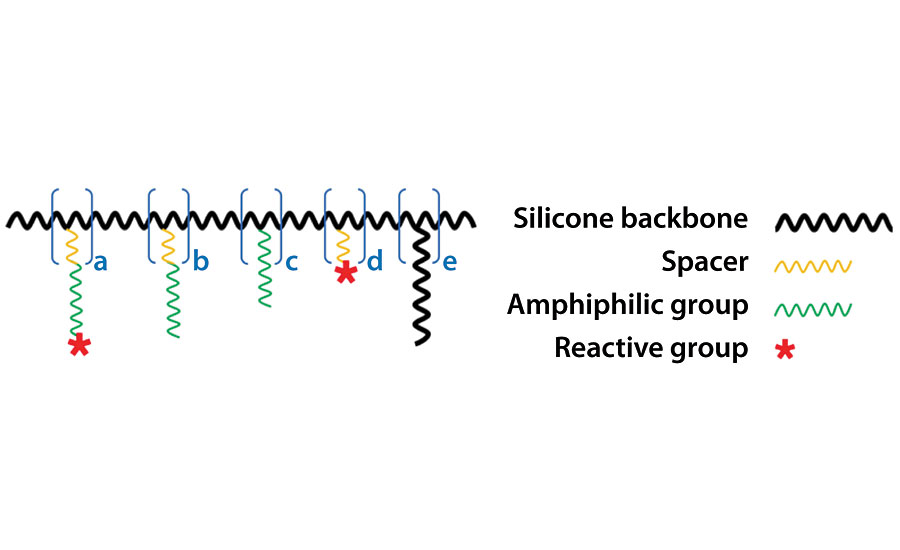
The SAP has a highly customizable, multi-functional brush-like molecular architecture that enables another level of modular approach (patent pending). The highly flexible polysiloxane backbone provides compatibility with the surrounding silicone matrix due to its chemical similarity, while its conformational freedom also promotes presenting the hydrophilic side chains towards the surface when immersed in an aqueous environment. The reactive group introduced at the end of a sidechain takes part in the crosslinking reaction of the polymer network formation, which effectively tethers the SAP and prevents it from leaching. When a lubricant of similar molecular structure to the SAP is introduced to the silicone matrices, the tethered SAPs effectively control the migration and release behavior of the lubricant due to increased compatibility. Inherently, however, SAPs and an amphiphilic lubricant are generally not fully compatible in a silicone matrix. Therefore, an initially homogenized mixture develops into a highly micro-phase-separated system (in both bulk and on the surface) via self-structuring processes driven by several mechanisms: 1) solubility mismatch-driven structuring, 2) diffusion and ripening process, 3) interfacial energy-driven structuring, 4) evaporative structuring and skin layer formation. Understanding and controlling the interplay between SAPs and the lubricant within a given binder system is a key to reproducibly successful formulation of non-toxic, non-biocidal antifouling paints. AST is currently examining an extensive library of proprietary SAPs and lubricants as a combination additive system called APIs (Active Performance Ingredients) mainly in silicone systems but also in some silicone-epoxy and silicone-urethane hybrid binder systems.
Speedy Product Development by Rapid Screening Tests
Implementing the hybrid modular approach in boat paint product development requires optimization of multiple parameters, since there are many possible combinations in materials used and in process features, even at the early formulation stage. Despite the emergence of data science and optimization tools, paint formulation development is still largely driven by empirical approaches. Thus, AST adopted a series of rapid screening tests to quickly downselect top candidates among numerous formulations tested. The development workflow is summarized in Figure 8, which begins from the design and synthesis of SAPs and lubricants, formulation prototyping with APIs and subsequent screening. First screening is carried out based on basic wetting and surface properties, then based on early lab-scale single species tests with toxicity, adherence and waterjet removal. Success there leads to promising formulations moving onto screening in AST’s own field test site in Port Canaveral, FL, chosen for its high fouling pressure. The “final” screened formulations are further validated for long-term field performance under both static and dynamic exposure conditions at various third-party global locations. In parallel, the downselected formulations are further productized for sagging-leveling balance, pot life, tolerance to curing conditions, pigmentation, and shelf life before going through the scale-up process.
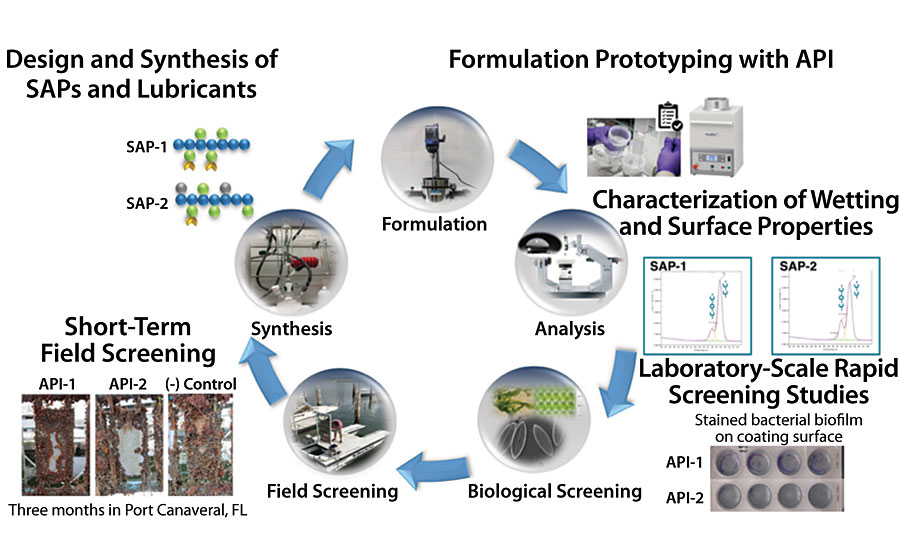
AST studies show that SLIPS products are non-toxic across biological organisms, as confirmed by cytotoxicity tests as well as leachate toxicity tests against a gram-negative marine bacterium (C. lytica) and diatom alga (N. incerta), while their adhesion is effectively mitigated as shown in water jet removal tests. Non-toxicity to barnacles (A. Amphitrite) and mussels (G. demissa and P. viridis) is also supported by healthy growth of these organisms without visible ill effects in completed laboratory studies, while also demonstrating extremely low adhesion of these common fouling organisms to AST marine coatings. (Figure 9).
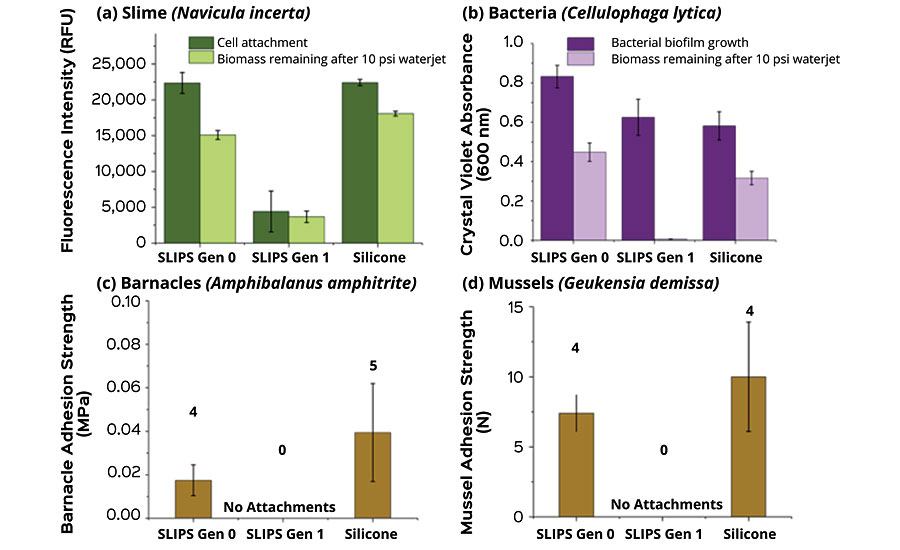
Fuel Saving and Eco-Friendly Marine Bottom Paints
The application of the API approach to a silicone system while providing desired properties as a paint (adhesion, durability, cost, application, usability) yielded the SLIPS Foul Protect™ marine paint product family. SLIPS N1x bottom paint, launched in April 2019, is a hybrid of Reservoir SLIPS and API package based on commercially available raw materials. Unlike the commonly used biocide-laden coatings lasting for only one season, N1x is designed for multiseason performance and does not release harmful chemicals to the environment. Through the support of the Department of Energy’s ARPA-E program, Foul Protect N1 (an earlier version of N1x) was tested on a Harbormaster patrol boat in Marion, MA. After six months of usage without any cleaning, the hull was found to be free of any hard fouling and was covered with only very light slime (Figure 10). The bottom could be quickly cleaned with a low-pressure water jet without using any chemicals and could be put back in the water with a fast turnaround time, which is critical for the boat owner.

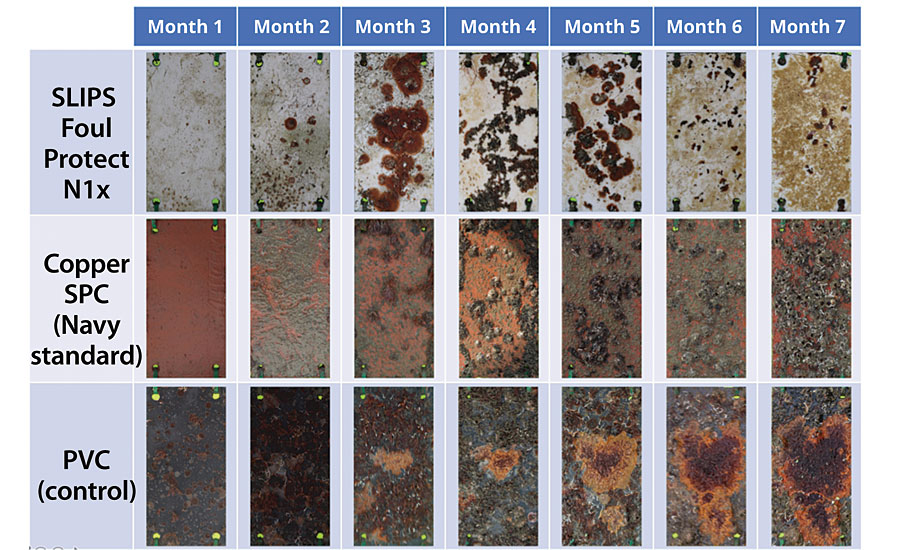
SLIPS Foul Protect coatings have shown a significant benefit in biofouling performance compared to copper-based SPC coating under static field test conditions in Port Canaveral, FL (Figure 11). This test site is considered to have aggressive biofouling pressure all year round with minimal seasonal effects, which allows performance screening for tough operating conditions. Using the model described by Schultz, 1,2 the biofouling performance benefit of the coatings enables about 8% more reduction in drag penalty compared to copper-based SPC coating after seven months of static exposure. AST has also launched an optically transparent version of marine paint, SLIPS SeaClear ® , which can be applied to submerged sensors and aquariums.
Conclusions and Outlook
AST’s SLIPS Foul Protect N1x has successfully demonstrated the pathway to reducing marine fouling with a non-toxic, environmentally friendly approach combining the smooth and slippery liquid-based surface (Physical module) and highly branched amphiphilic SAPs (Chemical module). In the future, carefully designed and tested APIs can be introduced as additives to any existing marine paint system or even other coating systems such as architectural paints, interior coatings, food or medical coatings, etc. to impart antifouling properties. Such systems can still utilize conventional antimicrobial or antifungal agents as a new hybrid approach, where as an added benefit, the concentration of active agents may be reduced when synergistic effect is achieved. AST is continuing to test its advanced formulations and expects to release new products in the near future.
Acknowledgments
We are grateful to ARPA-E of the US Department of Energy for the risk-taking decision to bridge an early-stage academic invention to a revenue-generating product (Award no. DE-AR0000759), the generous support for in-lab and field testing by the Office of Naval Research, in particular by Shane Stafslien at North Dakota State University, Dr. Serena Teo at National University of Singapore, and Dr. Dean Wendt and Grant Waltz at California Polytechnic State University. We also thank our consultants Market Entropy, Illara Consulting, and Safinah Group, the enthusiasm and support for boat testing done by Cooley Marine Management, LLC and Marion Harbormaster Boatyards, and discussions with Prof. Joanna Aizenberg and her group at Harvard University and Wyss Institute for Biologically Inspired Engineering.
References
1 Schultz, M.P. Effects of Coating Roughness and Biofouling on Ship Resistance and Powering. Biofouling, 23(5), 331-341 (2007).
2 Schultz, M.P.; Bendick, J.A.; Holm, E.R.; Hertel, W.M. Economic Impact of Biofouling on a Naval Surface Ship. Biofouling, 27(1), 87-98 (2011)
3 US Energy Information Administration - International Energy Outlook 2016, Chapter 8 Transportation sector energy consumption.
4 Aizenberg, J. Slippery Liquid-Infused Porous Surfaces. J. Ocean Tech., 9(4), 112-113 (2014).
5 Jelic-Mrcelic, G.; Sliskovic, M.; Antolic, B. Biofouling Communities on Test Panels Coated with TBT and TBT-Free Copper Based Antifouling Paints. Biofouling 22(5), 293-302 (2006).
6 Lejars, M.; Margaillan, A.; Bressy, C. Fouling Release Coatings: A Nontoxic Alternative to Biocidal Antifouling Coatings. Chem. Rev., 112(8), 4347-4390 (2012).
7 Baier, R.E. Surface Behaviour of Biomaterials: The Theta Surface for Biocompatibility. J. Mater. Sci: Mater. Med., 18, 1057-1062 (2006).
8 Frank, M. Antimicrobial Materials, Coatings and Biomimetic Surfaces with Modified Microtopography to Control Microbial Fouling of Product Contact Surfaces Within Food Processing Equipment: Legislation, Requirements, Effectiveness and Challenges. J. Hygien. Engr. Design, 7, 8-29 (2014).
9 Magin, C.M.; Cooper, S.P.; Brennan, A.B. Non-Toxic Antifouling Strategies. Materials Today, 13(4), 36-44 (2010).
10 Callow, J.A.; Callow, M.E. Trends in the Development of Environmentally Friendly Fouling-Resistant Marine Coatings. Nat. Commun., 2, 244 (2011).
11 Konstantinou, IK, Albanis, TA. Worldwide Occurrence and Effects of Antifouling Paint Booster Biocides in the Aquatic Environment: A Review. Environ. Int., 30(2) 235-248 (2004).
12 Wong, T.S.; Kang, S.H.; Tang, S.K.Y.; Smythe, E.J.; Hatton, B.D.; Grinthal, A.; Aizenberg, J. “Bioinspired Self-Repairing Slippery Surfaces with Pressure-Stable Omniphobicity. Nature, 477, 443-447 (2011).
13 Kim, P.; Wong, T.S.; Alvarenga, J.; Kreder, M.J.; Adorno-Martinez, W.E.; Aizenberg, J. “Liquid-Infused Nanostructured Surfaces with Extreme Anti-Ice and Anti-Frost Performance. ACS Nano, 6(8) 6569-6577 (2012).
14 Epstein, A.K.; Wong, T.S.; Belisle, R.A.; Boggs, E.M.; Aizenberg J. Liquid-Infused Structured Surfaces with Exceptional Anti-Biofouling Performance. PNAS 109(33), 13182-13187 (2012).
15 Kim, P.; Kreder, M.J.; Alvarenga, J.; Aizenberg, J. Hierarchical or Not? Effect of the Length Scale and Hierarchy of the Surface Roughness on Omniphobicity of Lubricant-Infused Substrates. Nano Lett., 13, 1793-1799 (2013).
16 Tesler, A.B.; Kim, P.; Kolle, S.; Howell, C.; Ahanotu, O.; Aizenberg, J. Extremely Durable Biofouling-Resistant Metallic Surfaces Based on Electrodeposited Nanoporous Tungstite Films on Steel. Nat. Commun., 6, 8649 (2015).
17 MacCallum, N.; Howell, C.; Kim, P.; Sun, D.; Friedlander, R.; Ranisau, J.; Ahanotu, O.; Lin, J.J.; Vena, A.; Hatton, B.; Wong, T.S.; Aizenberg, J. Liquid-Infused Silicone as a Biofouling-Free Medical Material. ACS Biomat. Sci. Eng. 1, 43-51 (2014).
18 Yao, X.; Dunn, S.S.; Kim, P.; Duffy, M.; Alvarenga, J.; Aizenberg, J. Fluorogel Elastomers with Tunable Transparency, Elasticity, Shape-Memory, and Antifouling Properties. Angew. Chem. Int. Ed., 53, 4418-4422 (2014).
19 Howell, C.; Vu, T.L.; Lin, J.J.; Kolle, S.; Juthani, N.; Watson, E.; Weaver, J.C.; Alvarenga, J.; Aizenberg, J. Self-Replenishing Vascularized Fouling-Release Surfaces. ACS Appl. Mater. Interf., 6, 13299-13307 (2014).
20 Amini, S.; Kolle, S.; Petrone, L.; Ahanotu, O.; Sunny, S.; Sutanto, C.N.; Hoon, S.; Cohen, L.; Weaver, J.C.; Aizenberg, J.; Vogel, N.; Miserez, A. Preventing Mussel Adhesion Using Lubricant-Infused Materials. Science 357, 668-673 (2017).
21 Kovalenko, Y.; Sotiri, I.; Timonen, J.V.I.; Overton, J.C.; Holmes, G.; Aizenberg, J.; Howell, C. Bacterial Interactions with Immobilized Liquid Layers. Adv. Healthcare Mat., 1600948 (2017).
22 Cassé, F.; Stafslien, S.J.; Bahr, J.A.; Daniels, J.; Finlay, J.A.; Callow, J.A.; Callow, M.E. Combinatorial Materials Research Applied to the Development of New Surface Coatings V. Application of a Spinning Water-Jet for the Semi-High Throughput Assessment of the Attachment Strength of Marine Fouling Algae. Biofouling, 23(2), 121-130 (2007).
23 Stafslien, S.J.; Daniels, J.; Mayo, B.; Christianson, D.; Chisholm, B.; Ekin, A.; Webster, D.C.; Swain, G. Combinatorial Materials Research Applied to the Development of New Surface Coatings IV. A High-Throughput Bacterial Biofilm Retention and Retraction Assay for Screening Fouling-Release Performance of Coatings. Biofouling, 23(1), 45-54 (2007).
24 Stafslien, S.J.; Daniels, J.; Bahr, J.; Chisholm, B.; Ekin, A.; Webster, D.C.; Orihuela, B.; Rittschof, D. An Improved Laboratory Reattachment Method for the Rapid Assessment of Adult Barnacle Adhesion Strength to Fouling-Release Marine Coatings. J. Coat. Technol. Res., 9 (6) 651-665 (2012).
25 Galhenage, T.P.; Webster, D.C.; Moreira, A.M.S.; Burgett, R.J.; Stafslien, S.J.; Vanderwal, L.; Finlay, J.A.; Franco, S.C.; Clare, A.S. Poly(ethylene) Glycol-Modified, Amphiphilic, Siloxane-Polyurethane Coatings and Their Performance as Fouling-Release Surfaces. J. Coat. Technol. Res., 14 (2) 307-322, (2017).
Looking for a reprint of this article?
From high-res PDFs to custom plaques, order your copy today!







